March 9, 2007 at the normal melting point.
advertisement
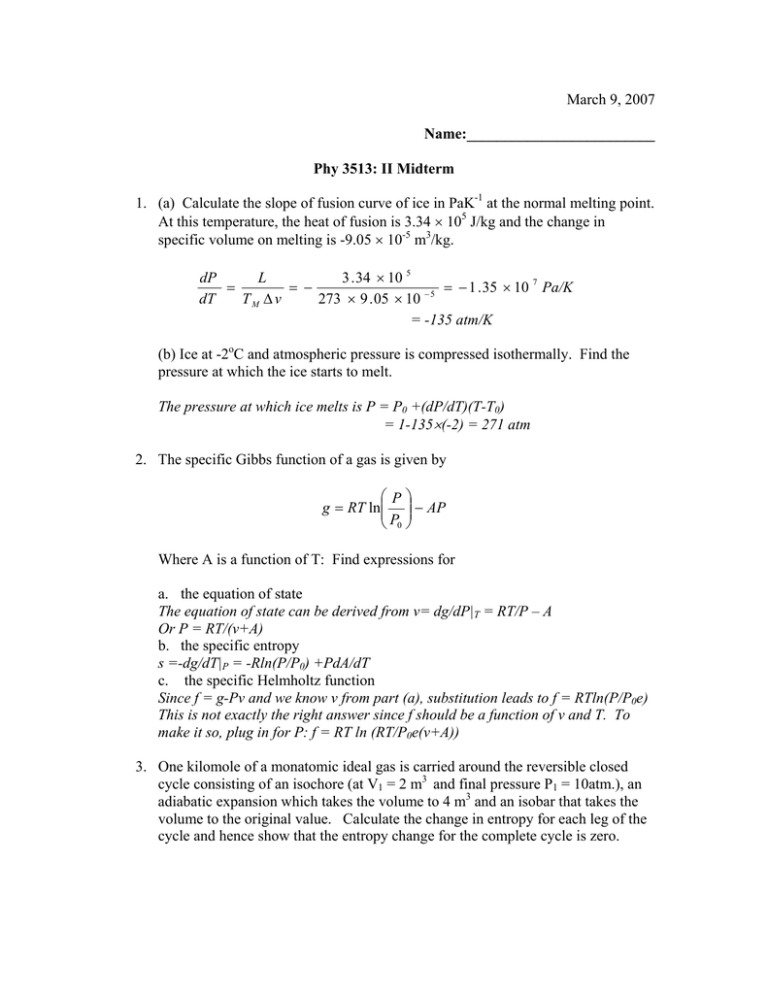
March 9, 2007 Name:_________________________ Phy 3513: II Midterm 1. (a) Calculate the slope of fusion curve of ice in PaK-1 at the normal melting point. At this temperature, the heat of fusion is 3.34 × 105 J/kg and the change in specific volume on melting is -9.05 × 10-5 m3/kg. 3 . 34 × 10 5 dP L = = − = − 1 . 35 × 10 7 Pa/K −5 273 × 9 . 05 × 10 dT TM Δ v = -135 atm/K (b) Ice at -2oC and atmospheric pressure is compressed isothermally. Find the pressure at which the ice starts to melt. The pressure at which ice melts is P = P0 +(dP/dT)(T-T0) = 1-135×(-2) = 271 atm 2. The specific Gibbs function of a gas is given by ⎛P⎞ g = RT ln⎜⎜ ⎟⎟ − AP ⎝ P0 ⎠ Where A is a function of T: Find expressions for a. the equation of state The equation of state can be derived from v= dg/dP|T = RT/P – A Or P = RT/(v+A) b. the specific entropy s =-dg/dT|P = -Rln(P/P0) +PdA/dT c. the specific Helmholtz function Since f = g-Pv and we know v from part (a), substitution leads to f = RTln(P/P0e) This is not exactly the right answer since f should be a function of v and T. To make it so, plug in for P: f = RT ln (RT/P0e(v+A)) 3. One kilomole of a monatomic ideal gas is carried around the reversible closed cycle consisting of an isochore (at V1 = 2 m3 and final pressure P1 = 10atm.), an adiabatic expansion which takes the volume to 4 m3 and an isobar that takes the volume to the original value. Calculate the change in entropy for each leg of the cycle and hence show that the entropy change for the complete cycle is zero. Cycle in P-V plane is shown in the figure. In the step 2-3, the process being adiabatic, there is no exchange of heat and consequently, the change in entropy is zero. Δs23 = 0. P 2 1 3 V ⎛T ⎞ Δ s 12 = c v ln ⎜⎜ 2 ⎟⎟ ⎝ T1 ⎠ ⎛T ⎞ Δ s 31 = c p ln ⎜⎜ 1 ⎟⎟ Since we know that V3/V1=2, P2 ⎝ T3 ⎠ = 10 atm, and cp = 5/2R (cv = 3/2R), we can calculate these numbers. To be complete, here is a table of numbers derived from known information. Point 1 Point 2 Point 3 Pressure (atm) 3.15 10 3.15 Volume (m3) 2 2 4 Δs12 = 3 ⎡ 243.69 ⎤ × 8314 × ln ⎢ = 1.44 × 10 4 J / Kkmole ⎥ 2 ⎣ 76.74 ⎦ Δs31 = 5 ⎡ 76.74 ⎤ × 8314 × ln ⎢ = −1.44 × 10 4 J / Kkmole ⎥ 2 ⎣153.48 ⎦ Δs = Δs12 + Δs31 + Δs23 = 0 Temperature(K) 76.74 243.69 153.48











