Capture of an Acid-Sensitive Recombinant Protein from E. coli
advertisement
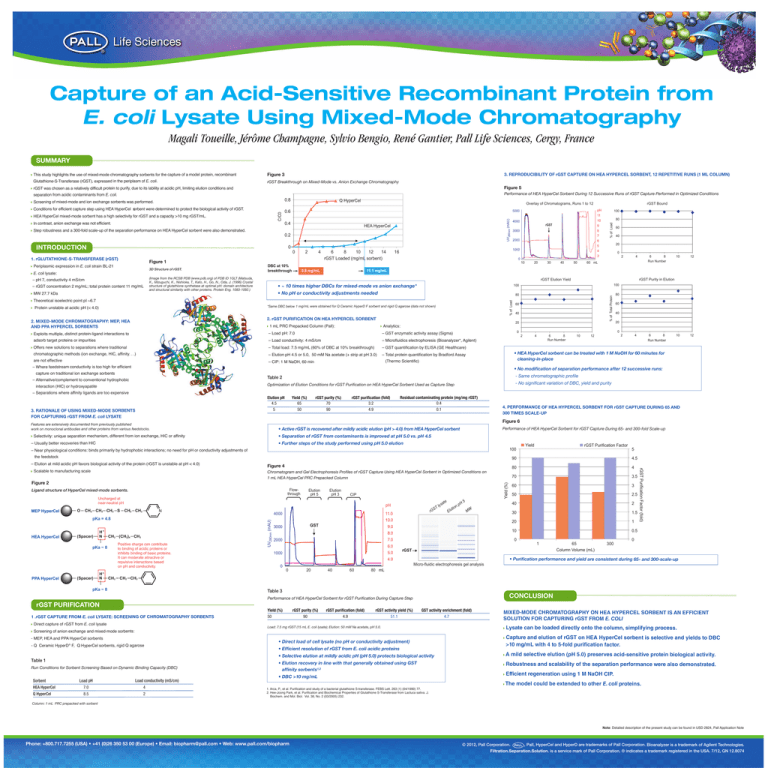
Capture of an Acid-Sensitive Recombinant Protein from E. coli Lysate Using Mixed-Mode Chromatography Magali Toueille, Jérôme Champagne, Sylvio Bengio, René Gantier, Pall Life Sciences, Cergy, France SUMMARY This study highlights the use of mixed-mode chromatography sorbents for the capture of a model protein, recombinant Figure 3 Glutathione-S-Transferase (rGST), expressed in the periplasm of E. coli. rGST Breakthrough on Mixed-Mode vs. Anion Exchange Chromatography 3. REPRODUCIBILITY OF rGST CAPTURE ON HEA HYPERCEL SORBENT, 12 REPETITIVE RUNS (1 ML COLUMN) rGST was chosen as a relatively difficult protein to purify, due to its lability at acidic pH, limiting elution conditions and Figure 5 separation from acidic contaminants from E. coli. Performance of HEA HyperCel Sorbent During 12 Successive Runs of rGST Capture Performed in Optimized Conditions HEA HyperCel mixed-mode sorbent has a high selectivity for rGST and a capacity >10 mg rGST/mL. In contrast, anion exchange was not efficient. 0.6 0.4 HEA HyperCel Step robustness and a 300-fold scale-up of the separation performance on HEA HyperCel sorbent were also demonstrated. 0.2 INTRODUCTION 0 0 4 Figure 1 Periplasmic expression in E. coli strain BL-21 3D Structure of rGST. E. coli lysate: – pH 7, conductivity 4 mS/cm – rGST concentration 2 mg/mL; total protein content 11 mg/mL 6 8 10 12 14 rGST Loaded (mg/mL sorbent) pH 100 4000 11 10 80 rGST DBC at 10% breakthrough 0.8 mg/mL 9 3000 8 7 2000 1000 16 0 10 (Image from the RCSB PDB (www.pdb.org) of PDB ID 1GLT (Matsuda, K., Mizuguchi, K., Nishioka, T., Kato, H., Go, N., Oda, J. (1996) Crystal structure of glutathione synthetase at optimal pH: domain architecture and structural similarity with other proteins. Protein Eng. 1083-1092.) 20 30 40 50 40 6 5 20 4 3 0 2 4 6 8 Run Number 60 mL 10 12 10 12 11.1 mg/mL • ~ 10 times higher DBCs for mixed-mode vs anion exchange* • No pH or conductivity adjustments needed % of Load Theoretical isoelectric point pI ~6.7 *Same DBC below 1 mg/mL were obtained for Q Ceramic HyperD F sorbent and rigid Q agarose (data not shown) rGST Purity in Elution 100 100 80 80 60 40 2. rGST PURIFICATION ON HEA HYPERCEL SORBENT 2. MIXED-MODE CHROMATOGRAPHY: MEP, HEA AND PPA HYPERCEL SORBENTS 60 rGST Elution Yield MW 27.7 kDa Protein unstable at acidic pH (< 4.0) 5000 % of Total Protein 1. rGLUTATHIONE-S-TRANSFERASE (rGST) 2 rGST Bound Overlay of Chromatograms, Runs 1 to 12 UV280nm (mAU) C/C0 Conditions for efficient capture step using HEA HyperCel sorbent were determined to protect the biological activity of rGST. Q HyperCel % of Load 0.8 Screening of mixed-mode and ion exchange sorbents was performed. 20 1 mL PRC Prepacked Column (Pall): Analytics: Exploits multiple, distinct protein-ligand interactions to – Load pH: 7.0 – GST enzymatic activity assay (Sigma) adsorb target proteins or impurities – Load conductivity: 4 mS/cm – Microfluidics electrophoresis (Bioanalyzer*, Agilent) Offers new solutions to separations where traditional – Total load: 7.5 mg/mL (60% of DBC at 10% breakthrough) – GST quantification by ELISA (GE Healthcare) chromatographic methods (ion exchange, HIC, affinity. . .) – Elution pH 4.5 or 5.0, 50 mM Na acetate (+ strip at pH 3.0) – Total protein quantification by Bradford Assay are not effective – CIP: 1 M NaOH, 60 min 60 40 20 0 0 2 4 6 8 Run Number 10 2 12 4 6 8 Run Number • HEA HyperCel sorbent can be treated with 1 M NaOH for 60 minutes for cleaning-in-place (Thermo Scientific) – Where feedstream conductivity is too high for efficient • No modification of separation performance after 12 successive runs: capture on traditional ion exchange sorbents – Alternative/complement to conventional hydrophobic interaction (HIC) or hydroxyapatite Table 2 - Same chromatographic profile Optimization of Elution Conditions for rGST Purification on HEA HyperCel Sorbent Used as Capture Step - No significant variation of DBC, yield and purity – Separations where affinity ligands are too expensive 3. RATIONALE OF USING MIXED-MODE SORBENTS FOR CAPTURING rGST FROM E. coli LYSATE rGST purity (%) 70 90 Yield (%) 65 50 Elution pH 4.5 5 rGST purification (fold) 3.2 4.9 Residual contaminating protein (mg/mg rGST) 0.4 0.1 4. PERFORMANCE OF HEA HYPERCEL SORBENT FOR rGST CAPTURE DURING 65 AND 300 TIMES SCALE-UP Figure 6 Features are extensively documented from previously published work on monoclonal antibodies and other proteins from various feedstocks. Performance of HEA HyperCel Sorbent for rGST Capture During 65- and 300-fold Scale-up • Active rGST is recovered after mildly acidic elution (pH > 4.0) from HEA HyperCel sorbent Selectivity: unique separation mechanism, different from ion exchange, HIC or affinity • Separation of rGST from contaminants is improved at pH 5.0 vs. pH 4.5 – Usually better recoveries than HIC • Further steps of the study performed using pH 5.0 elution Yield 100 – Near physiological conditions: binds primarily by hydrophobic interactions; no need for pH or conductivity adjustments of the feedstock – Elution at mild acidic pH favors biological activity of the protein (rGST is unstable at pH < 4.0) 90 4.5 Figure 4 80 4 Chromatogram and Gel Electrophoresis Profiles of rGST Capture Using HEA HyperCel Sorbent in Optimized Conditions on 1 mL HEA HyperCel PRC Prepacked Column 70 3.5 60 3 50 2.5 40 2 30 1.5 20 1 10 0.5 Flowthrough Ligand structure of HyperCel mixed-mode sorbents. Elution pH 5 Elution pH 3 Yield (%) Figure 2 CIP Uncharged at near-neutral pH CH2 CH2 CH2 S CH2 CH2 (Spacer) H+ N CH2 (CH2)4 CH3 pKa ~ 8 (Spacer) PPA HyperCel H+ N CH2 Positive charge can contribute to binding of acidic proteins or inhibits binding of basic proteins. It can moderate attractive or repulsive interactions based on pH and conductivity. T S G r 11.0 4000 pKa = 4.8 HEA HyperCel te pH UV280nm (mAU) O MEP HyperCel 5 a lys Elu t ion pH 5 MW 10.0 GST 3000 rGST Purification Factor (fold) Scalable to manufacturing scale rGST Purification Factor 9.0 8.0 0 7.0 2000 1 6.0 5.0 1000 0 rGST 65 Column Volume (mL) 300 • Purification performance and yield are consistent during 65- and 300-scale-up 4.0 Micro-fluidic electrophoresis gel analysis 0 0 20 40 60 80 mL CH2 CH2 pKa ~ 8 Table 3 CONCLUSION Performance of HEA HyperCel Sorbent for rGST Purification During Capture Step rGST PURIFICATION 1 .rGST CAPTURE FROM E. coli LYSATE: SCREENING OF CHROMATOGRAPHY SORBENTS Direct capture of rGST from E. coli lysate Yield (%) 50 rGST purity (%) 90 rGST purification (fold) 4.9 rGST activity yield (%) 51.1 GST activity enrichment (fold) 4.7 Load: 7.5 mg rGST (15 mL E. coli lysate); Elution: 50 mM Na acetate, pH 5.0. Screening of anion exchange and mixed-mode sorbents: - MEP, HEA and PPA HyperCel sorbents ® - Q Ceramic HyperD F, Q HyperCel sorbents, rigid Q agarose Table 1 Run Conditions for Sorbent Screening Based on Dynamic Binding Capacity (DBC) Sorbent Load pH Load conductivity (mS/cm) HEA HyperCel 7.0 4 Q HyperCel 8.5 2 • Direct load of cell lysate (no pH or conductivity adjustment) • Efficient resolution of rGST from E. coli acidic proteins Mixed-Mode chroMatography on hea hypercel sorbent is an efficient solution for capturing rgst froM E. COLI lysate can be loaded directly onto the column, simplifying process. capture and elution of rgst on hea hypercel sorbent is selective and yields to dbc >10 mg/ml with 4 to 5-fold purification factor. • Selective elution at mildly acidic pH (pH 5.0) protects biological activity a mild selective elution (ph 5.0) preserves acid-sensitive protein biological activity. • Elution recovery in line with that generally obtained using GST affinity sorbents1,2 robustness and scalability of the separation performance were also demonstrated. • DBC >10 mg/mL efficient regeneration using 1 M naoh cip. the model could be extended to other E. coli proteins. 1. Arca, P., et al. Purification and study of a bacterial glutathione S-transferase. FEBS Lett. 263 (1) (04/1990) 77. 2. Hee-Joong Park, et al. Purification and Biochemical Properties of Glutathione S-Transferase from Lactuca sativa. J. Biochem. and Mol. Biol. Vol. 38, No. 2 (03/2005) 232. Column: 1 mL PRC prepacked with sorbent Note: Detailed description of the present study can be found in USD 2824, Pall Application Note Phone: +800.717.7255 (USA) • +41 (0)26 350 53 00 (Europe) • Email: biopharm@pall.com • Web: www.pall.com/biopharm © 2012, Pall Corporation. , Pall, HyperCel and HyperD are trademarks of Pall Corporation. Bioanalyzer is a trademark of Agilent Technologies. Filtration.Separation.Solution. is a service mark of Pall Corporation. ® indicates a trademark registered in the USA. 7/12, GN 12.8074