Lattice Geometry Identification
advertisement

Lattice Geometry
Identification
Crystallographic Planes and Directions
X-Ray Diffraction
Material Sciences and Engineering
MatE271
Week4
1
Importance of Crystal Structures,
Directions, Planes?
o Properties depend on geometry of
crystals
• Speed of light, sound
• Strength
• Conductivity
o In cubic single crystals properties are
isotropic, all other systems are
anisotropic
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
2
1
Crystallographic Planes & Directions
direction
plane
o Many material properties and processes vary with
direction in the crystal
o It is often necessary to be able to specify certain
directions and planes in crystals.
o Directions and planes are described using three
integers - Miller Indices
Material Sciences and Engineering
MatE271
Week 4
3
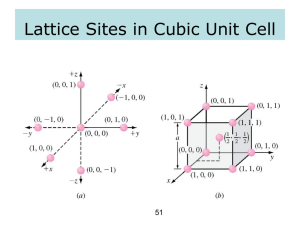
Indexing in 2D
o Determine ∆ in unit distances to move from one
lattice point to the next in the plane (or direction).
o Put in x,y format.
(10)
(11)
Properties:
Lowest Indices - Greatest plane spacing
Lowest Indices - Greatest density of lattice points
(21)
This is true in 3-D as well
(41)
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
(13)
MatE271
Week 4
4
2
General Rules for Lattice Directions, Planes
& Miller Indices
o Miller indices used to express lattice planes and
directions
o x, y, z are the axes (on arbitrarily positioned
origin)
• in some crystal systems these are not
mutually ⊥
o a, b, c are lattice parameters (length of unit cell
along a side)
o h, k, l are the Miller indices for planes and
directions - expressed as (hkl) and [hkl]
Material Sciences and Engineering
MatE271
Week 4
5
Miller Indices for Directions
o Conventions for naming
• There are NO COMMAS between numbers
• Negative values are expressed with a bar over
the number (-2 is expressed 2)
o Crystallographic direction:
• [123]
• [100]
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
6
3
Miller Indices for Directions
Recipe
• Draw vector, define tail as
origin.
• Determine length in unit
cell dimensions, a, b, and c
• Remove fractions by
multiplying by smallest
possible factor
• Enclose in square brackets
• What is ???
• x = 1/2, y = 0, z = 1
• [1/2 0 1] -> [1 0 2]
Material Sciences and Engineering
z
[???]
[111]
y
[100]
[110]
x
Entire lattice can be
referenced by one
unit cell!
MatE271
7
Week 4
Example - Naming Directions
z
z
[111]
[110]
y
x
z
x
z
x
[010]
y
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
[111]
y
[210]
x
z
y
z
y
x
[111]
y
x
MatE271
Week 4
8
4
Example - Drawing Directions
o Draw [112] [111] and [222]
1/2
1/2
Material Sciences and Engineering
MatE271
9
Week 4
Families of Directions
o Equivalence of directions
[101] = [110]
[101] ≠ [110]
tetragonal
cubic
o <123> Family of directions
• e.g.
[123], [213], [312], [132], [231]
• (only in a cubic crystal)
• In the cubic system directions having the same
indices regardless of order or sign are equivalent
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
10
5
Miller Indices for Planes
o (hkl) Crystallographic plane
o {hkl} Family of crystallographic planes
• e.g. (hkl), (lhk), (hlk) etc.
• In the cubic system planes having the same
indices regardless of order or sign are equivalent
o Hexagonal crystals can be expressed in a four
index system (u v t w)
• Can be converted to a three index system using
formulas
Material Sciences and Engineering
MatE271
11
Week 4
Miller Indices for Planes
Recipe
• If the plane passes through the
origin, select an equivalent
plane or move the origin
• Determine the intersection of
the plane with the axes in
terms of a, b, and c
• Take the reciprocal (1/∞ = 0)
• Convert to smallest integers
(optional)
• Enclose by parentheses
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
z
(111)
y
x
Note - plane // to axis,
intercept = ∞ and
1/∞ = 0
Week 4
12
6
Crystallographic Planes
z
z
(001)
z
y
x
z
y
x
(201)
x
z
y
z
(100)
(212)
y
x
(011)
(111)
y
x
Material Sciences and Engineering
y
x
MatE271
Week 4
13
X-Ray Diffraction
o Can be used to determine crystal structure
(and hence identity of an unknown material)
o Diffraction occurs whenever a wave
encounters a series of regularly spaced objects
that;
• Can scatter the wave
• Have a spacing comparable to the wavelength
o X-ray wavelength ~ inter-atomic spacing and
are scattered by atoms.
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
14
7
Constructive & Destructive Interference
Constructive
Maximum and
minimum results from
two diffracting beams
phase shifted from one
another.
Destructive
Material Sciences and Engineering
MatE271
Week 4
15
Bragg’s Law
o For constructive interference, the additional path length
SQ+QT must be an integral number of wavelengths: Real
diffraction is more complicated for non-simple cubic
X-ray Source:
Monochromatic
and in-phase
P
S
Q
T
nλ = SQ + QT = dhklsinθ + dhklsinθ = 2 dhklsin θ
n = 1,2,3…order of reflection
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
16
8
Bragg’s Law, Cubic Symmetry
o Real diffraction is more complicated for non-simple cubic
systems because some set’s of atoms (e.g. BCC center
atoms) can produce out of phase scattering at certain
Bragg angles θ. Net effect…some of the diffracted
beams, that according to Bragg’s Law should be present,
are cancelled out.
o Example - for diffraction to occur:
BCC - h + k + l must be even
FCC - h, k, l must all be either even or odd
o Magnitude of difference between two adjacent and
parallel planes of atoms is function of Miller Indices and
the lattice parameter. For cubic symmetry:
dhkl = a/(h2 + k2 + l2)1/2
Material Sciences and Engineering
MatE271
Week 4
17
Diffractometer Technique
o Use powder (or polycrystalline) sample to guarantee
some particles will be oriented properly such that every
possible set of crystallographic planes will be available for
diffraction.
o Each material has a unique set of planar distances and
extinctions, making X-ray diffraction useful in analysis of
an unknown.
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
18
9
Example
o For BCC Fe, compute
a) the interplanar spacing
b) the diffraction angle for (220) set of planes.
The lattice parameter for Fe is 0.2866 nm and the
wavelength used is 0.1790 nm. Consider 1st order
reflections only.
Material Sciences and Engineering
MatE271
Week 4
19
Reading Assignment
Shackelford 2001(5th Ed)
Read: pp 88, 101-110
Check class web site:
www.public.iastate.edu\~bastaw\courses\Mate271.html
Material Sciences and Engineering
Material Sciences and Engineering,
MatE271
MatE271
Week 4
2
20
10