Figure 9-7
advertisement

• The indeterminate situation arises because the plane passes
through the origin. After translation, we obtain intercepts
(1,1, ) .
• By inverting them, we get ( 1 10) .
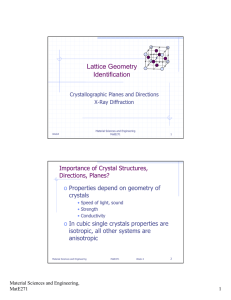
Stacking of (0002) planes
Figure 9-7 Hexagonal
structure consisting of
a three-unit cell.
Atoms in primitive cell
Additional atoms
[100]
• The third common metallic crystal structure is the hexagonal
close-packed (hcp) structure ( Fig.9-7).
• For hexagonal structures, we have slightly more complicated
situation.
• We represent the hexagonal structure by the arrangement
shown in Figure 9-7.
• The atomic arrangement in the basal plane is shown in the
top portion of the figure. Often, we use four axes (x, y, k, z)
with unit vectors (h, k , i, l ) to represent the structure.
• This is mathematically unnecessary, because three indices
are sufficient to represent a direction in space from a known
origin.
• Still, the redundancy is found by some people to have its
advantages and is described here.
• We use the intercepts to designate the planes.
• The hatched plane (prism plane) has indices.
1 1 1 1
, , ,
1 1
• After determining the indices of many planes, we learn that
one always has
h + k = -i
• Thus, we do not have to determine the index for the third
horizontal axis. If we use only three indices, we can use a
dot to designate the fourth index, as follows:
(1 1 0)
• For the directions, we can use either the three-index notation
or a four-index notation.
• However, with four indices, the h+k=-i rule will not apply
in general, and one has to use special “tricks” to make the
vector coordinates obey the rule.
• Crystallographic directions are indicated by integers
in brackets: [uvw]. Reciprocals are not used in
determining directions.
• For example, the direction of the line FD of Figure 9.1
is obtained by moving out from the origin a distance
of ao along the x axis and moving an equal distance in
the positive direction.
• The indices of this direction are then [ 110].
• A family of crystallographically equivalent directions
would be designated <uvw>.
• For the cubic lattice only, a direction is always
perpendicular to the plane having the same indices.
• The notation used for a direction is [uvw].
• When we deal with a family of directions, we use the symbol
<uvw>.
• The following family encompasses all equivalent directions:
uvw [uvw],[uwv ],[ wuv ],[ wvu ],[vuw],
[uv w],[uwv ],[ wuv ],[ wv u ],[v uw]
100 [100],[010],[001]
[ 1 00],[0 1 0],[00 1 ]
110 [110],[101],[011]
[ 1 1 0],[ 1 0 1 ],[0 1 1 ]
[1 1 0],[10 1 ],[01 1 ]
[ 1 10],[ 1 01],[0 1 1]
Figure 9-8 Various directions
in a cubic system.
• For cubic systems there is a set of simple relationships between a
direction [uvw] and a plane (hkl) which are very useful.
1) [uvw] is normal to (hkl) when u=h;v=k;w=l.
[111] is normal to (111).
2) [uvw] is parallel to (hkl), i.e., [uvw] lies in (hkl),
when hu + kv + lw = 0 [112] is a direction in (111).
3) Two planes (h1k1l1) and (h2k2l2) are normal if
h1h2 + k1k2 + l1l2 = 0. (100) is perpendicular to (001) and
(010). (110) is perpendicular to (110)
4) Two directions u1v1w1 and u2v2w2 are normal if
u1u2 + v1v2 + w1w2 = 0. [100] is perpendicular to
[001]. [111] is perpendicular to [112].
5) Angles between planes (h1k1l1) and (h2k2l2) are given by
h1h2 k1k2 l1l2
cos 2
(h1 k12 l12 )1/ 2 (h22 k22 l22 )1/ 2
Example: Write the indices of the marked planes
Figure 9-9
Answer:
Figure 9-9
Example: Write the indices of the marked directions
Figure 9-10
Answer:
Figure 9-10
Example: Write the indices of the marked planes and
directions
Figure 9-11
Answer:
Figure 9-11
Exercise:
Sketch the 12 members of the <110> family for a
cubic crystal. Indicate the four {111} planes.
You may use several sketches.
These are the 12 members of the <110> family of
directions for a cubic crystal.
These are the four members of the {111} family of
planes for a cubic crystal.