Sick Kids with an Unusual Organic Aciduria Problem 5 -
advertisement
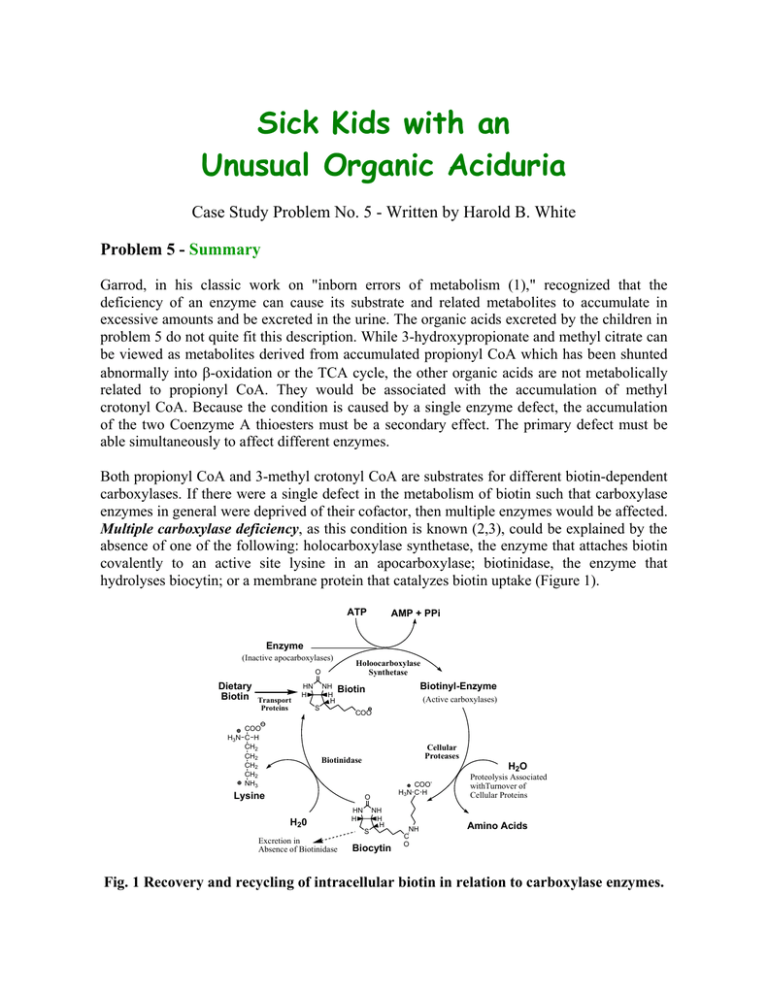
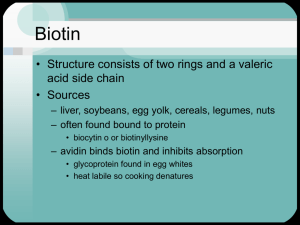
Sick Kids with an Unusual Organic Aciduria Case Study Problem No. 5 - Written by Harold B. White Problem 5 - Summary Garrod, in his classic work on "inborn errors of metabolism (1)," recognized that the deficiency of an enzyme can cause its substrate and related metabolites to accumulate in excessive amounts and be excreted in the urine. The organic acids excreted by the children in problem 5 do not quite fit this description. While 3-hydroxypropionate and methyl citrate can be viewed as metabolites derived from accumulated propionyl CoA which has been shunted abnormally into β-oxidation or the TCA cycle, the other organic acids are not metabolically related to propionyl CoA. They would be associated with the accumulation of methyl crotonyl CoA. Because the condition is caused by a single enzyme defect, the accumulation of the two Coenzyme A thioesters must be a secondary effect. The primary defect must be able simultaneously to affect different enzymes. Both propionyl CoA and 3-methyl crotonyl CoA are substrates for different biotin-dependent carboxylases. If there were a single defect in the metabolism of biotin such that carboxylase enzymes in general were deprived of their cofactor, then multiple enzymes would be affected. Multiple carboxylase deficiency, as this condition is known (2,3), could be explained by the absence of one of the following: holocarboxylase synthetase, the enzyme that attaches biotin covalently to an active site lysine in an apocarboxylase; biotinidase, the enzyme that hydrolyses biocytin; or a membrane protein that catalyzes biotin uptake (Figure 1). ATP AMP + PPi Enzyme (Inactive apocarboxylases) Holoocarboxylase Synthetase O Dietary Biotin Transport Proteins HN H COO H3N C H CH2 CH2 CH2 CH2 NH3 NH H H Biotinyl-Enzyme Biotin S (Active carboxylases) COO Cellular Proteases Biotinidase Lysine H2O COOH3N C H O H20 Excretion in Absence of Biotinidase HN H NH H H S Biocytin NH C O Proteolysis Associated withTurnover of Cellular Proteins Amino Acids Fig. 1 Recovery and recycling of intracellular biotin in relation to carboxylase enzymes. In humans, four carboxylases (pyruvate carboxylase, acetyl CoA carboxylase, propionyl CoA carboxylase, and 3-methyl crotonyl CoA carboxylase) account for almost all cellular biotin (4). Although pyruvate carboxylase and acetyl CoA carboxylase activities are low in multiple carboxylase deficiency, any accumulated pyruvate or acetyl CoA can be metabolized by well known reactions without conversion to unusual metabolites. The presence of lactic aciduria may be attributed to the disruption of pyruvate carboxylase and acetyl CoA carboxylase. By contrast there are no ready alternate ways to metabolize propionyl CoA or 3-methyl crotonyl CoA. As their concentrations rise, they become unnatural substrates for various enzymes and yield nonmetabolizable products that must be excreted. As with other coenzymes, Coenzyme A functions catalytically. The stoichiometric accumulation of nonmetabolizable CoA thioesters removes CoA from high flux pathways and can have disastrous consequences. Thioesterases and glycine conjugases provide ways to liberate CoA from these organic acids and recycle the CoA into other pathways. These reactions produce the unusual organic acids observed in multiple carboxylase deficiency. Therapy It has been shown that many patients with multiple carboxylase deficiency have defects in biotinidase (5-7). As a consequence, biotin is not recovered from biocytin and presumably is excreted. Thus, multiple carboxylase deficiency can be viewed as a genetically induced type of biotin deficiency. In order to replenish the biotin pools, patients are fed pharmacological doses of biotin (10 mg/day) and, happily, most recover and go on to live a normal symptom-free life. The accompanying photograph shows patient 1 at three years and one month of age, six months after the previous photograph and after four months of biotin therapy (2). Unfortunately, most metabolic diseases are not so easily treated. For example, if the holocarboxylase synthetase were defective, the consumption of large amounts of biotin would have little effect on multiple carboxylase deficiency. About 50% of the cases of human biotinidase deficiency are associated with a sevennucleotide deletion in the region coding for the signal peptide for biotinidase (8). Over 20 different mutations are associated with biotinidase deficiency (9). Recently two asymptomatic adults with biotinidase deficiency and different mutations from the above have been discovered (10). It is not understood why they are not affected. Biotin-dependent carboxylases (bold arrows) and the metabolic consequences (accumulation of unusual organic acids) of decreased biotin availability. Glycolysis ATP COO HO C H CH3 NAD+ L-Lactate COO C O CH2 COO Biotin COO C O CH3 Pyruvate Carboxylase Pyruvate HCO3- TPP Lipoamide FAD + Citric Acid Cycle Gluconeogenesis Oxaloacetate n io at tiv c a NADH+H +CO2 ATP SCoA C O CH3 COO CH2 CH2OH ADP+Pi ADP+Pi Biotin Acetyl CoA Carboxylase Acetyl CoA HCO3- SCoA C O CH2 COO Fatty Acid Synthesis Malonyl CoA 3-Hydroxypropionate CoASH Valine, Isoleucine Odd chain Fatty Acids ATP As in beta oxidation SCoA C O CH2 CH2OH SCoA C O CH CH2 COO H 3C C H HO C COO CH2 COO CoASH ADP+Pi Biotin SCoA C O CH2 CH3 Propionyl CoA Carboxylase HCO3- SCoA C O H C COO CH3 S-Methylmalonyl CoA SCoA C O CH2 CH2 COO Citric Acid Cycle Succinyl CoA Oxaloacetate Methylcitrate Leucine SCoA C O CH2 OH H3C CH3 CoASH COO CH2 C OH H3C CH3 3-Hydroxyisovalerate SCoA C O CH H3 C CH3 ATP ADP+Pi SCoA C O 3-Methyl CH Crotonyl CoA Carboxylase C H3C CH2 COO Biotin HCO3- Glycine CH2COO HN C O CoASH CH C H3C CH3 3-Methylcrotonylglycine HMGCoA Ketogenesis References Cited 1.Garrod, A. (1908) Inborn errors of metabolism (first of four lectures in the Croonian Lectures) The Lancet (7/4/08) pp.1-7. 2. Thoene, J. Baker, H., Yoshino, M. & Sweetman, L. (1981) Biotin-responsive carboxylase deficiency associated with subnormal plasma and urinary biotin. New England Journal of Medicine 304, 817-820. (Also see the commentary by K. Tanaka on pp. 839-840 of the same issue.) 3. Sweetman, L., Bates, S. P., Hull, D. & Nyhan, W. L. (1977) Propionyl CoA carboxylase deficiency in a patient with biotin responsive 3-methylcrotonylglycinuria. Pediatric Res. 11, 1144-1147. 4. Chandler, C. S. & Ballard, F. J. (1985) Distribution and degradation of biotin-containing carboxylases in human cell lines. Biochem. J. 232, 385-393. 5. Thoene, J. & Wolf, B. (1983) Biotinidase deficiency in juvenile multiple carboxylase deficiency. Lancet (8/13/83) p 398. 6. Wolf, B., Grier, R. E., Allen, R. J., Goodman, S. I. & Kien, C. L. (1983) Biotinidase deficiency: the enzyme defect in late-onset multiple carboxylase deficiency. Clinica Chimica Acta 131, 273-281. 7. Salbert, B. A., Pellock, J. M. & Wolf, B. (1993) Characterization of seizures associated with biotinidase deficiency. Neurology 43, 1351-1355. 8. Pomponio, R.J., Reynolds, T.R., Cole, H., Buck, G.A. & Wolf, B. (1995) Mutational hotspot in the human biotinidase gene causes profound biotinidase deficiency. Nat Genet 11, 96-98. 9. Pomponio, R.J., Hymes, J., Reynolds, T.R., Meyers, G.A., Fleischhauer, K., Buck, G.A., Wolf, B. (1997) Mutations in the human biotinidase gene that cause profound biotinidase deficiency in symptomatic children: molecular, biochemical, and clinical analysis. Pediatr Res 42, 840-848. 10. Wolf, B., Norrgard, K., Pomponio, R.J., Mock, D.M., McVoy, J.R., Fleischhauer, K., Shapiro, S., Blitzer, M.G. & Hymes, J. (1997) Profound biotinidase deficiency in two asymptomatic adults. Am J Med Genet 73, 5-9.