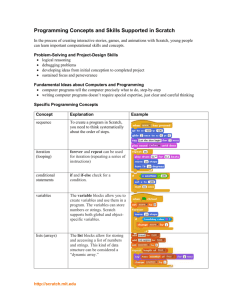
CHEM 332. Midterm 1

Name: _________________________________
CHEM 332. Midterm 1
Spring 2014
Prof Donald Watson
Please write your answers clearly in the boxes provided. If your answer is illegible or outside the box, it will not be graded. You may use the back of test pages for scratch work.
You may use molecular models.
Use of calculators, cell phones, headphones, or any other electronic device during this exam is prohibited.
No notes or books may be used during this exam.
You may raise your hand to ask a question if you are not sure what is being asked of you.
There are 10 pages in this exam. Please check that your test has 10 pages before you begin. The last 3 pages are blank and may be used as scratch paper.
Question Points
1
2
3
_____ /10
_____ /25
_____ /20
4
5
_____ /25
_____ /20
Total ____ /100
1
Name: _________________________________
1. (10 points) In the space below, please list all of the requirements for a molecule to be aromatic according to Huckel’s rules. Please number your list.
2
Name: _________________________________
2. (25 points, 5 points each) Please draw any significant product(s) expected for each of the following reactions. If more than one product is expected, please indicated relative ratio (major, minor, trace, etc). If no reaction is expected, write “No Reaction.”
Br
HNO
3
/H
2
SO
4 a) b)
Me
O O
Me O Me
AlCl
3 c)
Me
+
MeO
2
C
CO
2
Me Δ d)
Et
+
CN
Δ e)
Me
+
I OMe cat. Pd(PPh
3
)
4
, Et
3
N
3
Name: _________________________________
3. (20 points) In the space below, provide a mechanism for the following transformation.
Please include any important resonance structures that are important to the reaction progressing.
NO
2 NO
2
Cl
NaOMe, Δ OMe
4
Name: _________________________________
4. (25 points) Please propose a synthesis of 1,3-dibromobenzene starting from benzene. You can use any reagents and as many steps as you see fit, however, only concise routes will be given full credit.
Br
Br
5
Name: _________________________________
5. (20 points) Upon heating, the cyclopropyl cation below rearranges to the allyl cation shown (note the geometry with respect to the methyl groups). Using Woodward-
Hoffman correlation diagrams, please explain why this selectively forms this product.
Δ H Me
H
Me
H
Me
Me H
6
Name: _________________________________
If extra space is requires for problem 5, please use the space below.
7
Name: _________________________________
This page was intentionally left blank and may be used for scratch paper.
8
Name: _________________________________
This page was intentionally left blank and may be used for scratch paper.
9
Name: _________________________________
This page was intentionally left blank and may be used for scratch paper.
10