SCH4U Hess’s Law Page 326 # 1 - 3
advertisement

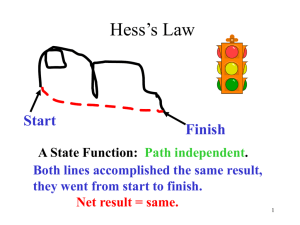
SCH4U Hess’s Law Additivity of Heats Page 326 # 1 - 3 329 # 4, 5 330 # 1 - 4 Herman Hess Potential energy of hiker 1 and hiker 2 is the same even though they took different paths. Hess's Law of Heat Summation The enthalpy change of an overall process is the sum of the enthalpy changes of its individual steps. Example: Problem: Calculate the energy involved in the oxidation of elemental sulfur to sulfur trioxide from reactions: 1) S (s) + O2 (g) SO2 (g) H1 = -296.0 kJ 2) 2 SO2 (g) + O2 (g) 2 SO3 (g) H2 = -198.2 kJ SO3 (g) H3 = ? Target equation: 3) S (s) + 3/2 O2 (g) If you add two or more equations to get a new equation, you must add the H's to get the the new equation. HRXN for Given: 1) S (s) + O2 (g) + ½[ 2 SO 2 (g) + O2 (g) S (s) + O 2 (g) + SO 2 (g) + ½ O2 (g) SO2 (g) 2 SO3 (g)] H1 = -296.0 kJ [ SO2 (g) + SO 3 H2 = -198.2 kJ] *½ H1 = -296.0 kJ + H2 = -198.2/2 kJ Find a substance that appears in only one place. Make sure that the substance is on the correct side of the targetequation. Add or subtract the equation. Get the same coefficient as the target equation Target equation: S (s) + 3/2 O 2 (g) SO3 (g) H3 = ? Thermochemical Equations • The stoichiometric coefficients always refer to the number of moles of a substance H2O (s) • DH = 6.01 kJ If you reverse a reaction, the sign of DH changes H2O (l) • H2O (l) H2O (s) DH = -6.01 kJ If you multiply both sides of the equation by a factor n, then DH must change by the same factor n. 2H2O (s) 2H2O (l) DH = 2 x 6.01 = 12.0 kJ Given: 1) S (s) + O2 (g) + 2) 2 SO2 (g) + O2 (g) S (s) + O 2 (g) + SO 2 (g) + ½ O2 (g) S (s) + 3/2 O 2 (g) SO2 (g) H1 = -296.0 kJ 2 SO3 (g) H2 = -198.2 kJ SO2 (g) + SO 3 SO3 (g) H1 = -296.0 kJ + H2 = -198.2/2 kJ H3 = -296 - 99.1 kJ H3 = -395.1 kJ Simplify to get the target equation and ÄH3. Target equation: S (s) + 3/2 O 2 (g) SO3 (g) H3 = ? Thermochemical Equations • The physical states of all reactants and products must be specified in thermochemical equations. H 2O (s ) H 2O ( l) H 2O H 2O ( l) D H = 6.01 kJ (g ) D H = 44.0 kJ Determine the heat of reaction for the reaction: 4NH3(g) + 5O2(g) à 4NO(g) + 6H2O(g) Using the following sets of reactions: N2(g) + O2(g) à 2NO(g) DH = 180.6 kJ N2(g) + 3H2(g) à 2NH3(g) DH = -91.8 kJ 2H2(g) + O2(g) à 2H2O(g) DH = -483.7 kJ The three reactions must be algebraically manipulated to sum up to the desired reaction. and.. the DH values must be treated accordingly. Target: 4NH3(g) + 5O2(g) à 4NO(g) + 6H2O(g) Using the following sets of reactions: N2(g) + O2(g) à 2NO(g) DH = 180.6 kJ N2(g) + 3H2(g) à 2NH3(g) DH = -91.8 kJ 2H2(g) + O2(g) à 2H2O(g) DH = -483.7 kJ Find a substance that appears in only one place. O2 : Found in more than one place, SKIP IT (its hard). Make sure that the substance is on the correct side of the targetequation. Target: 4NH3(g) + 5O2(g) à 4NO(g) + 6H2O(g) Using the following sets of reactions: N2(g) + O2(g) à 2NO(g) DH1 = 180.6 kJ N2(g) + 3H2(g) à 2NH3(g) DH2 = - 91.8 kJ 2H2(g) + O2(g) à 2H2O(g) DH3 = - 483.7 kJ Make sure that the substance is on the correct side of the targetequation. Reverse equation 2 and change the sign ofÄH2 2NH3(g) à N2(g) + 3H2 (g) DH2 = + 91.8 kJ Get the same coefficient as the target equation Target: 4NH3(g) + 5O2(g) à 2*[ N2(g) + O2(g) à 2NO(g) 2*[ 2NH3(g) 4NO(g) + 6H2O(g) ] à N2(g) + 3H2 (g) ] 3*[2H2(g) + O2(g) à 2H2O(g) ] 2*[ DH1 = 180.6 kJ ] 2*[ DH2 = + 91.8 kJ ] 3*[ DH3 = - 483.7 kJ ] DH1 = 2(180.6 kJ) 2N 2(g) + 2O 2 (g) à 4NO(g) +4NH 3 (g) +2N 2 (g) + 6H2 (g) + 2(91.8 kJ) +3(- 483.7 kJ) + 6H2(g) + 3O2(g) +6H 2 O(g) 4NH3(g) + 5O2(g) à 4NO(g) + 6H2O(g) DH = - 906.3 kJ Get the same coefficients as the target equation Goal: NH3: O2 : NO: H2O: 4NH3(g) + 5O2(g) à 4NO(g) + 6H2O(g) Reverse and x2 4NH3 à 2N2 + 6H2 DH = Found in more than one place, SKIP IT. x2 x3 2N2 + 2O2 à 4NO 6H2 + 3O2 à 6H2O +183.6 kJ DH = 361.2 kJ DH = -1451.1 kJ Cancel terms and take sum. 4NH3 + 5O2 à 4NO + 6H2O DH = -906.3 kJ Is the reaction endothermic or exothermic? Determine the heat of reaction for the reaction: C2H4(g) + H2(g) à C2H6(g) Use the following reactions: C2H4(g) + 3O2(g) à 2CO2(g) + 2H2O(l) DH = -1401 kJ C2H6(g) + 7/2O2(g) à 2CO2(g) + 3H2O(l) DH = -1550 kJ H2(g) + 1/2O2(g) à H2O(l) DH = -286 kJ . Determine the heat of reaction for the reaction: Goal: C2H4(g) + H2(g) à C2H6(g) DH = ? Use the following reactions: C 2H 4(g) + 3O 2(g) à 2CO 2(g) + 2H 2O(l) DH = -1401 kJ C 2H 6(g) + 7/2O 2(g) à 2CO 2 (g) + 3H2O(l) DH = -1550 kJ H 2(g) + 1/2O2(g) à H 2O(l) DH = -286 kJ C 2H 4(g) :use 1 as is C 2H 4(g) + 3O 2(g) à 2CO 2(g) + 2H 2O(l) H 2(g) :# 3 as is H 2(g) + 1/2O 2(g) à H 2O(l) C 2H 6(g) : rev #2 2CO 2(g) + 3H 2O(l) à C 2H6(g) + 7/2O2(g) C 2H 4(g) + H 2(g) à C 2H 6(g) DH = -1401 kJ DH = -286 kJ DH = +1550 kJ DH = -137 kJ Calculate the standard enthalpy of formation of CS 2 (l) given that: C(graphite) + O2 (g) CO2 (g) DH0rxn = -393.5 kJ S(rhombic) + O2 (g) CS2(l) + 3O2 (g) SO2 (g) DH0rxn = -296.1 kJ CO2 (g) + 2SO2 (g) 0 = -1072 kJ DHrxn 1. Write the enthalpy of formation reaction for CS2 C(graphite) + 2S(rhombic) CS2 (l) 2. Add the given rxns so that the result is the desired rxn. C(graphite) + O2 (g) 2S(rhombic) + 2O2 (g) + CO2(g) + 2SO2 (g) CO2 (g) DH0rxn = -393.5 kJ 2SO2 (g) DH0rxn = -296.1x2 kJ CS2 (l) + 3O2 (g) 0 = +1072 kJ DHrxn C(graphite) + 2S(rhombic) CS2 (l) DH0rxn= -393.5 + (2x-296.1) + 1072 = 86.3 kJ Benzene (C 6H6) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49.04 kJ/mol. 2C6H6 (l) + 15O2 (g) 12CO2 (g) + 6H2O (l) DH0rxn = S nDH0f (products) - S mDHf0 (reactants) DH0rxn = [ 12DH0f (CO2) + 6DH0f (H2O)] - [ 2DH0f (C6H6)] DH0rxn = [ 12x–393.5 + 6x–285.8 ] – [ 2x49.04 ] = -6534.88 kJ -6535kJ = - 3267 kJ/mol C6H6 2 mol 6.5 Sample Problem 6.6 PROBLEM: Using the Heat of Reaction (DHrxn) to Find Amounts The major source of aluminum in the world is bauxite (mostly aluminum oxide). Its thermal decomposition can be represented by Al2O3(s) 2Al(s) + 3/2O2(g) DHrxn = 1676kJ If aluminum is produced this way, how many grams of aluminum can form when 1.000x10 3kJ of heat is transferred? PLAN: SOLUTION: heat(kJ) 1676kJ=2molAl mol of Al xM g of Al 1.000x10 3kJ x 2mol Al 26.98g Al 1676kJ 1mol Al = 32.20g Al Sample Problem 6.7 PROBLEM: Using Hess's Law to Calculate an Unknown DH Two gaseous pollutants that form auto exhaust are CO and NO. An environmental chemist is studying ways to convert them to less harmful gases through the following equation: CO(g) + NO(g) CO2(g) + 1/2N2(g) DH = ? Given the following information, calculate the unknown DH: Equation A: CO(g) + 1/2O2(g) Equation B: N2(g) + O2(g) PLAN: CO2(g) DHA = -283.0kJ 2NO(g) DHB = 180.6kJ Equations A and B have to be manipulated by reversal and/or multiplication by factors in order to sum to the first, or target, equation. SOLUTION: Multiply Equation B by 1/2 and reverse it. CO(g) + 1/2O2(g) NO(g) CO(g) + NO(g) CO2(g) DHA = -283.0kJ 1/2N2(g) + 1/2O2(g) DHB = -90.6kJ CO2(g) + 1/2N2(g) DHrxn = -373.6kJ Dr. Schambaugh, of the University of Oklahoma School of Chemical Engineering, Final Exam question for May of 1997. Dr. Schambaugh is known for asking questions such as, "why do airplanes fly?" on his final exams. His one and only final exam question in May 1997 for his Momentum, Heat and Mass Transfer II class was: "Is hell exothermic or endothermic? Support your answer with proof." Most of the students wrote proofs of their beliefs using Boyle's Law or some variant. One student, however, wrote the following: "First, We postulate that if souls exist, then they must have some mass. If they do, then a mole of souls can also have a mass. So, at what rate are souls moving into hell and at what rate are souls leaving? I think we can safely assume that once a soul gets to hell, it will not leave. Therefore, no souls are leaving. As for souls entering hell, let's look at the different religions that exist in the world today. Some of these religions state that if you are not a member of their religion, then you will go to hell. Since there are more than one of these religions and people do not belong to more than one religion, we can project that all people and souls go to hell. With birth and death rates as they are, we can expect the number of souls in hell to increase exponentially. Now, we look at the rate of change in volume in hell. Boyle's Law states that in order for the temperature and pressure in hell to stay the same, the ratio of the mass of souls and volume needs to stay constant. Two options exist: If hell is expanding at a slower rate than the rate at which souls enter hell, then the temperature and pressure in hell will increase until all hell breaks loose. If hell is expanding at a rate faster than the increase of souls in hell, then the temperature and pressure will drop until hell freezes over. So which is it? If we accept the quote given to me by Theresa Manyan during Freshman year, "that it will be a cold night in hell before I sleep with you" and take into account the fact that I still have NOT succeeded in having sexual relations with her, then Option 2 cannot be true...Thus, hell is exothermic." The student, Tim Graham, got the only A.