Analytical Ultracentrifugation of Nanoparticles of Nanoparticles
advertisement

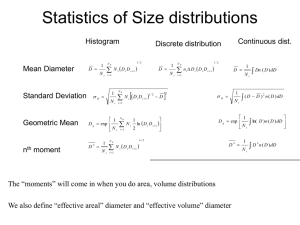
Analytical Analytical Ultracentrifugation Ultracentrifugation of of Nanoparticles Nanoparticles Helmut Cölfen Max-Planck-Institute of Colloids and Interfaces, Colloid Chemistry, Am Mühlenberg 2, D-14424 Potsdam Email: Coelfen@mpikg-golm.mpg.de Why Why fractionating fractionating analytics analytics in in solution solution ?? Light Light Scattering Scattering Microscopy Microscopy AUC AUC // FFF FFF No fractionation Counting of particles Drying artifacts Problem: Statistics Sensitive to bigger particles / dust as I∼r6 Problem: Big particles Absorption multiple scattering Fractionation Every particle is detected, simultaneous multiple detection possible Svedbergs Svedbergs Colloid Colloid Research Research • T. Svedberg, J.B. Nichols, J. Amer. Chem. Soc. 45 (1923) 2910 • T. Svedberg, H. Rinde, J. Amer. Chem. Soc. 45 (1923) 943 • T. Svedberg, H. Rinde, J. Amer. Chem. Soc. 46 (1924) 2677 • T. Svedberg, Kolloid-Z. Zsigmondy Festschrift, Erg.-Bd. Zu 36 (1925) 53 1925: Nobel prize for colloid work 1926: First protein work Principle Principle of of AUC AUC Radial Detection Light Solution Vapour ω ϕ h rm rt Detector r UV/VIS Absorption optics Δn Abs. Rayleigh Interference optics r rb r Experiments very simple, evaluation not always Sedimentation velocity High centrifugal force Sedimentation stronger than back diffusion Absorbance Different Different AUC AUC experiments experiments 1.0 0.5 50000 RPM Scaninterval 10 min 0.0 6.2 6.4 6.6 6.8 7.0 7.2 Sedimentation equilibrium Moderate centrifugal force Sedimentation in the order of back diffusion Absorbance Radius (cm) 2.0 Sedimentation 1.5 Diffusion 1.0 0.5 0.0 10000 RPM 6.7 6.8 6.9 7.0 7.1 Radius (cm) Density gradient High centrifugal force for distribution of a low molecular salt or a second solvent Absorbance 1.0 0.8 50 % CH2Cl2 + 50 % CHCl3 60000 RPM 0.6 0.4 0.2 0.0 6.2 6.4 6.6 6.8 Radius (cm) 7.0 7.2 Lamm equation Lamm equation ⎛ ⎞ ⎟ ∂c 1 d ⎜ dc 2 2 ⎜ rD = − sω r c ⎟ 1 424 3 ⎟ ∂t r dr ⎜ 123 dr Se dim entation term ⎟ ⎜ Diffusion term ⎝ ⎠ Experiment Effective term in the Lamm equation Characteristics Sedimentation velocity Sedimentation term much bigger than diffusion term High rotational speed Synthetic boundary experiment for the determination of D Only diffusion term effective Synthetic boundary cell, low rotational speed Sedimentation velocity Sedimentation and diffusion term effective / equilibrium between sedimentation and diffusion Moderate / low rotational speed Density gradient (special case of sedimentation equilibrium) Sedimentation and diffusion term effective / equilibrium between sedimentation and diffusion Moderate / high rotational speed, locally dependent solution density Absorbance Basic Basic evaluation evaluation important important for for nanoparticles nanoparticles 1 step means 1 component 1.0 0.5 50000 RPM Scaninterval 10 min 0.0 6.2 6.4 6.6 6.8 7.0 Flat baseline indicates purity 7.2 Radius (cm) a) Determine distance travelled in given time interval b) Calculate sedimentation coefficient s or its distribution c) Calculate particle size or distribution u s= 2 ωr s= ln(r /rm ) ω2 t 18ηs i di = ρ2 − ρ Sedimentation Sedimentation velocity velocity RT sRT 18ηs i di = M= f= ρ2 − ρ N AD D (1 − vρ ) Sedimentation velocity depends on: Molar mass / particle size Density Shape / Friction Charge for given exptl. parameters (temperature, solvent density & viscosity etc.) Particle size distributions calculated on a hard sphere basis Sedimentation Sedimentation velocity velocity 1.6 a) 1.4 1.845 1.2 1.0 Data for individual boundaries Regression line sw = 480 S 1.840 s= 0.8 ln(r /rm ) 0.6 ln(r/rm) Absorbance Gold in H2O at 5000 RPM, 25 °C 1.850 ω t 2 1.835 1.830 0.4 1.825 0.2 6.0 a) Raw data 6.2 6.4 6.6 6.8 7.0 7.2 b) 1.820 4.00E+008 ωt g*(s) c(s) diffusion corrected c) 1.0 1.0 18ηs i ρ2 − ρ di = d) 0.8 g*(d) g*(s) resp. c(s) 0.8 d) ddistribution 8.00E+008 2 1.2 b) Sevaluation c) sdistribution 6.00E+008 Radius [cm] 0.6 0.6 0.4 0.4 0.2 0.2 0.0 0 200 400 600 sedimentation coefficient [S] 800 0.0 6.0 6.2 6.4 6.6 6.8 Diameter [nm] 7.0 7.2 Common Common Problems Problems • Extremely broad s-distributions, Big aggregates or small impurities are not detected • Colloids aggregate or grow during centrifugation (concentration dependent aggregation) • Density of hybrid colloids is unknown to access particle size • Electrostatic stabilization complicates analysis due to charge contributions • Particle polydispersity in size, shape, density and hydration • High particle density often makes density gradients or density variation methods impossible • Often multicomponent mixtures Fractionation of heterogeneous samples Fractionation of heterogeneous samples 5 Carrageenan Absorbance / Fringe shift Absorption optics + iron oxyhydroxide Interference optics 4 Large aggregates Carrageenan + iron oxyhydroxide 3 0.55 fringes Free Carrageenan 2 2.72 fringes 1 40000 RPM, 25 °C 0 6,0 6,2 6,4 6,6 6,8 7,0 Protected partially mobile FeOOH Radius [cm ] pH 2, κ-carrageenan Hollow space + Fe3+ Fe3+ Fe3+ Fe3+ 3+ pH 2, Fe + OHpH > 5 crosslinking of κ-carrageenan Hydrolized Fe3+ F. Jones Latex Latex mixture mixture 1 .0 A. Völkel 60 nm 100 nm 0 .8 164 nm i Σc /c 0 0 .6 389 nm 0 .4 0 .2 3400 nm 0 .0 100 d= • 18 η s ρP − ρLM 1000 d H [μ m ] Particles analyzed over colloidal range in one experiment given d [nm] % 60 20 103 20 160 20 347 20 2800 20 reproduced d [nm] % 60 20 100 20 164 18 389 20 3400 22 Very Very small small colloids colloids Au [P( Phe ) ] Cl Au55 [P(Phe) ] 3 12 55 3 12Cl66 Reported to be definite cluster with 55 Au, Transition between metal and molecule 1530 counts 0.21 Expt. Data Sum Gauss Fit Ind. Gauss Fit g(dH) [A.U.] 0.14 Species 1: 1.84 +/- 0.15 nm (48.7 %) Species 2: 1.95 +/- 0.20 nm (26.0 %) Species 3: 2.34 +/- 0.32 nm (25.3 %) 0.07 0.00 1.5 2.0 2.5 dH [nm] 3.0 1.0 High High resolution resolution PSD PSD 0.9 Absorbance 0.8 0.7 0.6 0.5 0.4 Pt colloid in MeOH / HAc 0.3 0.2 60000 RPM, 25 °C, 380 nm Scanint. 2 min 0.1 0.0 6.0 6.2 6.4 6.6 6.8 7.0 7.2 Radius (cm) 1.0 0.1 nm Baseline resolution 1.77 nm 1.6 1.8 1.92 nm 1.59 nm 1.68 nm 0.6 1.50 nm Differential 1.40 nm 0.8 1.30 nm Sum of mass fractions Integral 0.4 0.83 nm 0.2 0.0 0.4 0.6 0.8 1.0 1.2 1.4 Particle diameter [nm] Problem: Elimination of diffusion broadening by selfsharpening effects is not common 2.0 Baseline resolution > 1 Angström TEM TEM Number frequency % 20 350 counts 15 10 5 0 0.8 1.2 1.6 2.0 2.4 2.8 3.2 3.6 particle diameter [nm] Particles slightly bigger than from AUC (Density) Pt -Colloid growth Pt-Colloid growth Coalescence Critical crystal nucleus = Stabilizer = Pt-Particle Growth Coalescence until particles Further growth through are stabilized reduction of PtCl4 at the crystal surface dr = const. Differences in the particle size after coalescence are conserved dt Mechanism in G.A. Braun, Ph-D dissertation, Aachen 1997 Start of heating defines start of the reaction 5 0 2 4 6 8 10 Parti cle d 12 14 iame ter [n m] Problem: AUC has a low time resolution 300 250 200 150 100 50 tim e[ mi n] Dilution 1 : 5 and Heating to 65 °C 10 Re ac tio n Zn(Ac)2 (1 mmol/l) + 20 mmol/l NaOH in water free Isopropanol ts Arbitrary uni Growing Growing ZnO ZnO Colloid Colloid T. Pauck Bio -labeling Bio-labeling Au Au Au S Au S COO COO Differential distribution + BSA etched + transferred to water attached to protein 0 2 4 6 8 10 Particle size (nm) 12 D.I.Gittins and F.Caruso Phase -labeling Phase transfer transfer and and bio bio-labeling Au particles in toluene Au particles in water BSA labeled Au particles in water 1.0 0.8 Phase transfer agent: 11Mercaptoundecanoic acid (MUA) g(s) 0.6 0.4 0.2 0.0 0 500 1000 s25,w [S] 1500 2000 D.I.Gittins and F.Caruso Dopamine Dopamine functionalized functionalized TiO TiO22 0.10 3 0.09 Absorption g (d) 0.08 0.07 0.06 2 0.05 0.04 3 1 Particle size decreases 4 5 6 7 8 9 dH (nm) Spectra taken at different positions in distribution 0 200 250 300 350 400 450 500 550 600 Wavelength [nm] M. Niederberger Concept Concept of of turbidimetric turbidimetric immunoassay immunoassay Antibody (high reactivity) Antibody (low reactivity) Antigen 127 nm Latex particle 221 nm Latex particle Concept Concept of of turbidimetric turbidimetric immunoassay immunoassay Big particles aggregate first, then the small ones TEM TEM of of Immunoassay Immunoassay 0 mg/L CRP 4.28 mg/L CRP Statistics, drying artifacts ? 25 mg/L CRP 156 mg/L CRP Scale bars 500 nm SLS SLS of of Immunoassay Immunoassay 100 50 mg/L CRP G(dH) 80 • Fast measurement without equilibration needs • Kinetic measurements possible • Ambient conditions without any pressure effects • Low resolution in particle size • Low statistical resolution (No fractionation) 60 0 min 2 min 5 min 7 min 11 min 15 min 40 20 0 0.1 1 10 dH [μm] 100 G(dH) 80 171 mg/L CRP 60 0 min 2 min 4 min 7 min 11 min 15 min 40 20 0 0.1 1 dH [μm] 10 92 wt-% small latices AUC AUC of of Immunoassay Immunoassay 1.0 G(dH) 0.8 25 °C 0.6 • AUC has equilibration time > 10 min before experiment • Fractionation enables to determine correct particle quantities and sizes by turbidity detection (MIE correction) with speed profile even over decades of svalues • 92 wt-% small latices in mixture correctly detected 0.4 Latex mixture + 4.28 mg/L CRP + 50 mg/L CRP + 156 mg/L CRP 0.2 0.0 0.0 0.5 1.0 1.5 dH [μm] 2.0 2.5 3.0 1.0 G(dH) 0.8 0.6 Latex mixture + 5 mg/L CRP + 10 mg/L CRP + 20 mg/L CRP + 30 mg/L CRP + 40 mg/L CRP + 50 mg/L CRP 0.4 0.2 37 °C 0.0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 dH [μm] 1.4 1.6 1.8 2.0 2.2 Microgels Microgels Figure from: H.G. Müller, A. Schmidt, D. Kranz; Progr. Colloid Polym. Sci. 86, 70 (1991) b ⋅ d ρ P − ρs Q= ⋅ s 18 ⋅ η 2 h 3 b = mass ratio of soluble parts PNIPAM PNIPAM Microgels Microgels Fringe shift of crosslinked part: 7.90 Fringe shift of free chains : 2.37 10 76% 24% 4 Crosslinked 10000 rpm Not crosslinked 60000 rpm 8 6 4 Fringe shift Fringe shift 3 7,50 2 2,37 1 2 0 6.0 6.2 6.4 6.6 r [cm] 6.8 7.0 7.2 0 6.3 6.6 6.9 r [cm] D. Kuckling 1.0 1.0 0.8 0.8 0.6 0.6 G(Q) G(s) Swelling Swelling degree degree distribution distribution 0.4 0.2 0.2 Deswollen (40 °C) Swollen (25 °C) low crosslinking degree high crosslinking degree 0.0 0 200 400 600 800 s [Svedberg] ⎛ s unswollen ⎞ ⎟⎟ Q = ⎜⎜ ⎝ s swollen ⎠ 3 1000 0.4 1200 0.0 0 5 10 15 20 25 30 35 40 Q In principle also possible with soluble fraction b b ⋅ d 2h ρ P − ρs Q= ⋅ s 18 ⋅ η D. Kuckling Solvent Solvent density density variation variation PS123-P4VP145 in toluene resp. d-toluene 0,8 0.6 1,5 ρ [g/cm³] 0.8 2,0 1,0 ρTdT for the following data: ============================== low density:PS-P4VP in toluene high density:PS-P4VP in tol/tol-d8 Integral distribution integral distribution 1.0 0,6 18 η s d= ρP − ρLM 0.4 0,4 0.2 lower density higher density 0.0 0 100 200 300 400 sedimentation coefficient [S] 1,0 500 0,5 0,2 0,0 0,0 40 60 80 100 dH [nm] Combination of 2 s-distributions in 2 chemically identical solvents yields particle size and density distribution • • • Density in good agreement with values from density meter Particle size in agreement with results from DLS or viscosity measurements Same result for core crosslinked micelles K. Schilling PS -PMAA Latex PS-PMAA Latex mixture mixture 40 40 :: 60 60 1,0 integral psd differential psd 0,8 ρP = 1.188 g/ml nP= 1.460 b = 61.5% 0,6 0,4 ρP = 1.054 g/ml nP = 1.6 b = 38.5% 0,2 0,0 0 50 100 150 18( s2 ηs2− s1 ηs1 ) d= ρ s1 − ρ s2 200 250 300 350 400 450 s1 ηs1 ρs2 − s2 ηs2 ρs1 ρ = p s 1 η s1 − s2 η s2 A. Völkel Synthetic Synthetic hybrid hybrid colloids colloids 200 nm A B 200 nm Brushite CaHPO4 * 2H2O TG 18% Mineral C 100 nm A: Star like crystals/whisker, d = 17 nm, l = 184 nm B: Transversal growth, destabilization of filaments, further densification of core C: Compact aggregates with periodicity („Chrysanthemun“ structure) M. Breulmann Synthetic Synthetic hybrid hybrid colloids colloids whisker • Superposition of particle size and density distribution • Densities can exceed 2 g/ml 1.0 dense structure 0.6 0.4 0.2 400 300 200 0.0 0 10 20 30 s* [S] 40 tim e[ h] g(s*) 0.8 100 50 60 70 0 Synthetic Synthetic hybrid hybrid colloids colloids density increase PEG-PMAA-C12 1.0 2+ PEG-PMAA-C12 + Ca "Chrysanthemum" 1 week Diameter(DLS) = 150 nm g(s*) 0.8 polydispersity increase 0.6 • Global Analysis could reveal density and molar mass 0.4 0.2 0.0 0 5 10 15 20 25 30 s* [Svedberg] 35 • Clear monitoring of density increase upon Ca2+ complexation 40 Combination of AUC with DLS or better Fl-FFF is highly desirable Particle Particle size size and and density density CdS UV : d = 1.65 nm DLS : d = 1.6 nm „ AUC: d = 1.25 nm“ (s = 3.6 S) ρparticle = 3.2 g/cm3 dparticle = 1.6 nm calculated with ρCdS = 4.83 g/cm3 3 0,03 UV 2 1 0 200 250 300 350 400 450 λAbs [nm] 18ηs ρp = 2 + ρ s dp r*d(r) [a.u.] I [a.u.] 4 0,02 DLS 0,01 0,00 1 10 2 r [nm] Density core and shell are known With independent size measurement, hybrid particle is fully characterized dtotal = 1.6 nm dcore = 1.1 nm dshell = 0.5 nm (Lit.: dshell = 0.4 nm) Hybrid Hybrid Colloids Colloids Ferrihydrite Fe2O3 . x H2O • Iron storage protein Ferritin forms robust hollow sphere from 24 dimeric subunits • • Ferritin oligomerizes • Simultaneous particle size and density distribution • • Size and amount of oligomers ? Different amounts of ferrihydrite inside the core (up to 4500 Fe) Amount of ferrihydrite inside the core for the different oligomers ? A. Völkel Ferritin Ferritin Fl-FFF separates only according to particle size, AUC according to particle size and density 4.35x10-7cm2s-1 Dimer t0 = 0.455min AUC 0,8 g(s*) Signal [a.u.] 1,0 Fl-FFF Monomer Diffusion corrected g(s*) Sum of Gauss Fits Individual Gauss Fits 0,6 0,4 2.93x10-7cm2s-1 Trimer 2.29x10-7cm2s-1 0,2 0,0 0 2 4 6 8 10 12 14 16 18 20 22 t [min] ret 0 20 40 60 80 100 120 140 160 s* [S] AUC no baseline separation of oligomers (density distribution) Global analysis Monomer Dimer Trimer s* at 25 °C (AUC) 59.8 S 96.9 S 127.9 S D* at 25 °C (Fl-FFF) 3.68 x 10-7 cm2/s 2.41 x 10-7 cm2/s 1.90 x 10-7 cm2/s dH (Fl-FFF) 11.9 nm 18.1 nm 23.0 nm ρP 1.764 g/cm3 1.531 g/cm3 1.436 g/cm3 Mw 586,700 g/mol 954,100 g/mol 1,367,400 g/mol Oligomer amount 69.4 wt.-% 19.5 wt.-% 11.1 wt.-% n Fe3+ 1476 667 34 Diffusion corrected g(s*) Sum of Gauss Fits Individual Gauss Fits 3+ Ferritin low Fe content 1.0 3+ Ferritin high Fe content 0,8 0.8 0,6 0.6 g (s) g(s*) 1,0 0,4 0.4 0,2 0.2 0,0 Fe c o rri t se nte in w di nt ith m ha h en s ta di ighe tio ffe r F n be rent e 3+ ha vio ur 0.0 0 20 40 60 80 s* [S] 100 120 140 160 0 100 200 300 400 s (S) 500 600 700 800 First First direct direct observation observation of of near near critical critical size size clusters clusters by by AFM AFM S.T. Yau, P.G. Vekilov „Quasi-planar nucleus structure in apoferritin crystallization“, Nature 406, 494 - 497; 3 August 2000 σ = 1.1 Crystallization Molecules that appeared after capture of the previous frame Dissolution Molecules that are missing in the next frame D.W. Oxtoby „Catching crystals at birth“, Nature 406, 464 - 465; 3 August 2000: „Although the first small crystallites involved in nucleation form in the bulk of the solution, Yau and Vekilov can observe them only once they have fallen to the bottom of the vessel and attached themselves to the surface there. How can the authors be sure that they are observing the critical early stages of crystallization described by nucleation ?“ Synthetic Synthetic boundary boundary cell cell of of the the Vinograd Vinograd Type Type Reservoir Capillaries Reactant ( ) containing solution is layered onto the second reactant via the capillary ( ) Formation of a sharp reaction boundary L. Börger Reaction zone Particle Particle formation formation in in aa synthetic synthetic boundary boundary cell cell 1 Generation 2 Generation 1) Formed particles sediment out of the reaction zone, reaction is quenched 2) Particles diffuse back into the reaction zone, further growth 3) Sedimentation time >> reaction time N-th Generation Sedimentation of particles Transformation of a time distribution into a radial distribution according to size, Determination of particle size distribution possible Synthetic boundary crystallization ultracentrifugation 1.0 [Cd4SR10]20.5 nm g(dH) CdS + thio0.8 glycerin 0.6 [CdSR4]20.3 nm 0.4 0.2 8 exptl. Scans Sum Gauss Fit Individual Gauss Fits [Cd10S4SR16]41.0 nm Structure unknown literature 1.3 nm Cd17S4SR26 1.4 nm Structure unknown literature 1.6 nm Cd32S14SR36 1.8 nm 0.0 0.4 0.8 1.2 1.6 2.0 dH [nm] All known stable CdS growth species simultaneously accessible in one experiment. Even subcritical clusters detectable in solution Conclusion Conclusion • AUC is a very versatile instrument for colloid chemistry (Range of possible methods still not fully explored !!!) • Fractionation allows the investigation of complex mixtures or very polydisperse samples with s ranging over decades (speed profile) • • • High statistical accuracy Critical crystal nuclei and subcritical clusters can be resolved Crystal growth reactions can be investigated by transformation of time distribution into radial distribution BUT: • Colloids can express various unfavourable properties which makes them problematic to investigate • Fast multidetection systems together with global analysis can potentially adress even most complex mixtures with particle size and VBAR distribution but role of charge still unadressed Future Future Perspectives Perspectives • Fast speed profiles and detectors to suppress diffusion broadening • • Multi-detection systems (RI, UV-Vis, SLS and others) • New ultracentrifugation methods could allow to access new quantities Global Analysis (promising with SEDVEL & SEDEQUIL + DLS & Fl-FFF) to obtain s, M, D & VBAR distributions Surface tension from phase transfer, particle charge determination via sedimentation in pH or charge gradient, Conformational changes in solvent quality gradients, new synthetic boundary techniques for membrane / crystal growth investigations or chemical reactions in the AUC, etc. Recommended Recommended reading reading . Svedberg, K.O. Pedersen, The ultracentrifuge, Clarendon Press, Oxford (1940) he classical textbook about analytical ultracentrifugation still with much impact today .K. Schachman, Ultracentrifugation in biochemistry, Academic Press, New York (1959) compact and useful book covering experimental and theoretical aspects .E. Harding, A.J. Rowe and J.C. Horton; Analytical ultracentrifugation in biochemistry and polymer science, The royal society of chemistry, Cambridge, ISBN 0-85186-345-0 (1992) he most comprehensive modern book about analytical ultracentrifugation. A very good overview about methods & techniques of analytical ultracentrifugation and a valuable source of modern applications.