N t
advertisement
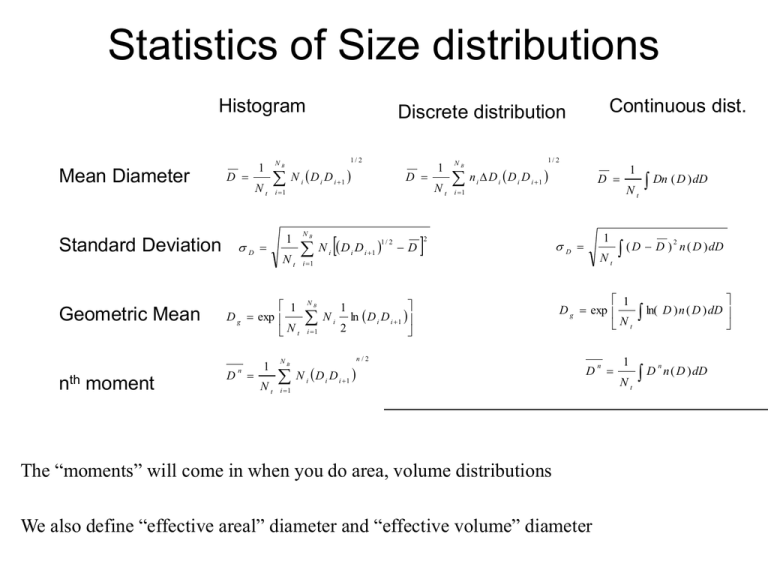
Statistics of Size distributions Histogram Mean Diameter Standard Deviation Geometric Mean D Nt D moment 1/ 2 NB N D D i D 1 Nt D i 1 i N D D NB 1 1/ 2 i Nt NB i 1 Nt i 1 i 1 D 1/ 2 NB n D D D i i i Nt i 1 2 NB i 1 N i ln D i D i 1 2 1 1 D Nt D i 1 The “moments” will come in when you do area, volume distributions We also define “effective areal” diameter and “effective volume” diameter n Dn ( D ) dD (D D ) 1 D g exp Nt n/2 N i D i D i 1 1 D i 1 i 1 1 D g exp Nt n nth 1 Continuous dist. Discrete distribution 1 Nt 2 n ( D ) dD ln( D ) n ( D ) dD D n n ( D ) dD Effective Diameters Consider Nt aerosol particles, each with Diameter D. This is a “monodisperse” distribution. 2 S N T D The Total surface area and volume will be: V NT D 3 6 Now consider the total surface area and volume of a polydisperse aerosol population 2 S D n( D)dD V Da D n( D)dD S D n( D)dD NT D 2 V 6 D n( D)dD NT 3 6 2 D 3 2 Dv D 3 6 Substituting our definition for moments, we have D 3 1/ 3 Effective Diameters Substituting our definition for moments, we have S NT D V NT 2 D 3 6 We now define an effective areal diamater, Da, and an effective volumetric diameter, Dv, which are the diameters that would produce the same surface area and volume if the distribution were monodisperse. Da D 2 Dv D 3 2 3 So… Board Illustration: Consider a population of aerosols where 900 cm-3 are 0.1 mm, and 100 cm-3 are 1.0 mm. Compute Da, Dv. S N T Da 2 V NT 6 3 Dv Converting size distributions Concentration: D i 1 N i ni Di D i 1 n ( D ) dD Di dN ( D ) Di Rule of thumb: Always use concentration, not number distribution, when converting from one type of size distribution to another dN ( D ) n ( D ) dD n (log D ) d log D n (ln D ) d ln D o e NOT n ( D ) n (log D ) n (ln D ) o e Converting size distributions Example: Let n(D) = C = constant. What are the log-diameter and ln-diameter distributions? dN ( D ) n ( D ) dD CdD n (ln D ) d ln D e dD n (ln D ) C e CD Even though n(D) is constant w/ diameter, the log distributions are functions of diameter. d ln D n (log D ) n (ln D ) 0 e d ln D d log D CD ln 10 2 . 303 CD Problem: Let n(D) = C = constant. What is the volumetric number distribution, dN/dv? The Power-Law (Junge) Size Distribution n(D)=C D-a n(D)=1000 cm-3 mm2 D-3 Linear-linear plot What is the total concenration, Nt? What is volume distribution, dV/dD? What is total volume? What is log-number distribution? Major Points for Junge Distr. Log-Log plot slope = -3 Need lower+upper bound Diameters to constrain integral properties Only accurate > 300 nm or so. Linear in log-log space…. The log-normal Size Distribution Note that power-law is simply linear in log-log space, and was unbounded y mx b ln n a ln D ln C n CD a Let’s make a distribution that is quadratic in log-log space (curvature down) y nx mx b 2 ln n ln D 2 2 a ln D ln C ln D 2a ln D n C exp 2 2 ln D a C exp 2 2 n (ln D ) e 2 a ln D ln D g 2 C 2 exp 2 2 n(D ) Nt 2 g 1 ln D ln D g 2 exp 2 g 2 2 ln D ln D Nt 1 g exp 2 g 2 g D 2 The Log-Normal Size Distribution n(D ) Nt=1000 cm-3, Dg = 1 mm, g = 1.0 1 ln D ln D g 2 exp 2 g 2 g D 2 Nt What is the total concenration, Nt? Linear-linear plot What is volume distribution, dV/dD? What is total volume? What is log-number distribution? Major Points for Log-normal 3 parameters: Nt, Dg and g Log-Log plot No need for upper/lower bound constraints goes to zero both ways Usually need multiple modes. More Statistics Medians, modes, moments, and means from lognormal distributions Median – Divides population in half. i.e. median of # distribution is where half the particles are larger than that diameter. Median of area distribution means half of the area is above that size Mode – peak in the distribution. Depends on which distribution you’re finding mode of (e.g. dN/dlogD or dN/dD). Set dn(D)/dD = 0 Secret to S+P 8.7… Get distributions in form where all dependence on D is in the form exp(-(lnD – lnDx)2). Complete the squares to find Dx. Then, the median and mode will be at Dx due to the symmetry of the distribution The means and the moments are properties of the integral of the size distribution. In the form above, these will appear outside the exp() term. (i.e. what is leftover after completing the squares). n (ln D ) Nt e n(D ) 2 g 1 ln D ln D g 2 exp 2 g 2 1 ln D ln D g 2 exp 2 g 2 g D 2 Nt n(D ) / 4 e n (ln D ) Standard 3-mode distributions Typical measured/parameterized urban size distributions Southern AZ size distributions Vertical distributions Often aerosol comes in layers Averaged over time, they form an exponentially decaying profile w/ scale height of ~1 to 2 km. Particle Aerodynamics S+P Chap 9. Need to consider two perspectives • Brownian diffusion – thermal motion of particle, similar to gas motions • Forces on the particle – Body forces: Gravity, electrostatic – Surface forces: Pressure, friction Relevant Scales • • Diameter of particle vs. mean free path in the gas – Knudsen # Inertial “forces” vs. viscous forces – Reynolds # Knudsen # Kn 2 = mean free path of air molecule Dp Dp = particle diameter Gas molecule selfcollision cross-section 1 2 B N B Gas # concentration Quantifies how much an aerosol particle influences its immediate environment • Kn Small – Particle is big, and “drags” the air nearby along with it • Kn Large – Particle is small, and air near particle has properties about the same as the gas far from the particle Kn Free Molecular Regime Transition Regime Continuum Regime