Thermally Activated Processes - Arrhenius Behavior - Diffusion:
advertisement

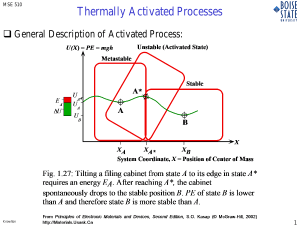
MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes General Description of Activated Process: Unstable (Activated State) U(X) = PE = mgh Metastable Stable E A ΔU A* U A* UA A U B B X XA XA* XB System Coordinate, X = Position of Center of Mass Fig. 1.27: Tilting a filing cabinet from state A to its edge in state A* requires an energy EA. After reaching A*, the cabinet spontaneously drops to the stable position B. PE of state B is lower than A and therefore state B is more stable than A. Knowlton From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002) http://Materials.Usask.Ca MSE 310 Elec. Props of Matls Knowlton 1 Thermally Activated Processes - Arrhenius Behavior - Diffusion: A A* B U = PE(x) U A* A* EA UA= UB A B Displacement X Fig. 1.28: Diffusion of an interstitial impurity atom in a crystal from one void to a neighboring void. The impurity atom at position A must posses an energy EA to push the host atoms away and move into the neighboring void at B. Knowlton From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002) http://Materials.Usask.Ca 2 1 Thermally Activated Processes - Arrhenius Behavior - MSE 310 Elec. Props of Matls Knowlton Diffusion: Thermally Activated Processes 9 Temperature Plays a significant role in diffusion 9 Temperature is not the driving force. 9 Remember: DRIVING FORCE = GRADIENT of a FIELD VARIABLE 9 Remember: Driving force for diffusion is a difference in the chemical potential. μ phase1 ≠ μ phase 2 i.e. Δμ ≠ 0 or ∇μ ≠ 0 9 HOWEVER: Temperature increases the activity of a diffusing species. 9 Question: What is the probability that an atom will diffuse? o Atoms are Bosons o Use Maxwell-Boltzmann Statistics! f (ε ) = N (ε )d ε = f (ε ) g (ε )d ε = = Ne − βε g (ε )d ε ∫e − βε g (ε )d ε Ne− βε ∫e − βε g (ε )d ε No. Impurities with E > E A Total No. of Impurities = Ae − EA kT Knowlton 3 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Diffusion: O' After N jumps L Y a θ = 90° θ = 0° θ = 180° O y X x θ = 270° Fig. 1.29: An impurity atom has four site choices for diffusion to a neighboring interstitial vacancy. After N jumps, the impurity atom would have been displaced from the original position at O. Knowlton From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002) http://Materials.Usask.Ca 4 2 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Diffusion: 9 Frequency of Jump is proportional to the probability 9 Coefficient of Diffusion is proportional to Jump Frequency ν = ν 0 f (ε ) = Aν 0 e − D ∝ν E kT ∝ Aν 0 e − E kT 9 Diffusivity or Diffusion Coefficient (Arrhenius Rate Equation): Equation) D = Do e − EA kT • Eact is the activation energy for diffusion • kb T is the thermal energy • Do, the pre-exponential factor, contains a number of physical constants and properties including: » » » » entropy of formation of the defect attempt frequency for jumps into available neighboring sites lattice constant crystal structure dependence Knowlton 5 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Diffusion: Thermally Activated Processes 9 Diffusivity or Diffusion Coefficient: D = Do e Knowlton − EA kT 6 3 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Diffusion: Diffusivity or Diffusion Coefficient: 9 Question: Question How does one obtain: ln D o Activation energy o Pre-exponential D = Do e − Eact m=− EA kB k BT Linearize Diffusivity: − Eact ⎛ ⎞ ln D = ln ⎜ Do e kBT ⎟ ⎝ ⎠ Best way to calculate E A : E = − A + ln Do k BT m= Has form of Equation of a Line! y = mx + b Thus: y = ln D; x = Δy ln D2 − ln D1 = 1 1 Δx − T2 T1 1 T So : E A = −k B ⋅ slope 1 E ; m = − A ; b = ln Do T kB = −kB ⋅ ln D2 − ln D1 1 1 − T2 T1 Knowlton 7 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - KINETICS: Diffusion and "Chemical Reactions: Thermally Activated Processes 9 We will use Si as an example of a system with various diffusion mechanisms. 9 Two types of diffusion mechanisms: o Direct diffusion mechanisms: diffusion without the aid of point defects. • Interstitial diffusion o Indirect diffusion mechanisms: diffusion with the aid of point defects As + V ⇔ AV Vacancy mechanism As + I ⇔ AI Interstitialcy mechanism As + I ⇔ Ai Kick-out mechanism As ⇔ Ai + V Dissociative (Frank-Turnbull) mechanism Knowlton 8 4 Thermally Activated Processes - Arrhenius Behavior - MSE 310 Elec. Props of Matls Knowlton KINETICS: Diffusion and "Chemical Reactions: Thermally Activated Processes 9 Chemical reaction causes a change in concentration, CI, of interstitials kf As + I ↔ AI kr ∂C ∂t reac I = kr C AI − k f CI C As k r & k f: forward & reverse Coefficients of reaction k = ko e EA kT 9 For the equation that describes the total kinetics, that is, the total change in the concentration of C, or Ctotal: ∂Ctotal ∂Cdiff ∂Crctn = + ∂t ∂t ∂t Knowlton 9 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Extra notes for those that are interested Arrhenius behavior is observed in many areas of science 9 Conduction in solids Knowlton 10 5 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Extra notes for those that are interested Other Examples of Arrhenius Rate Behavior: 9 Much of Kinetics shows this behavior o o o o o o Carrier concentration and conduction in semiconductors and insulators Mass Transport Defect Formation Rates of Chemical Reactions (Coefficient of Reaction Rate) Creep Rate Dislocation motion Knowlton 11 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Carrier Concentration in Semiconductors: Intrinsic EeEeeEc Ec e- e- Ee- e- e- e- ee- e- e- e- Ev Ev Ec e- Intrinsic Ee- e- Ed Ec n-type e- Ed Ev n-type Knowlton 12 6 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Carrier Concentration: n = no e − EG 2 k BT Intrinsic carrier concentration as a function of 1/T (Arrhenius plot). Knowlton 13 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Carrier Conductivity in insulators: 1×10-1 σ ∝n − EA As3.0Te3.0Si1.2Ge1.0 glass 1×10-3 k BT Conductivity 1/(Ωm) σ =σe 24%Na2O-76%SiO2 Pyrex 1×10-5 1×10-7 12%Na2O-88%SiO2 1×10-9 1×10-11 PVAc SiO2 PVC 1×10-13 1×10-15 1.2 1.6 2 2.4 2.8 103/T (1/K) 3.2 3.6 4 Fig. 2.28: Conductivity vs reciprocal temperature for various low conductivity solids. (PVC = Polyvinyl chloride; PVAc = Polyvinyl acetate.) Data selectively combined from numerous sources. From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002) http://Materials.Usask.Ca Knowlton 14 7 Thermally Activated Processes - Arrhenius Behavior - MSE 310 Elec. Props of Matls Knowlton SiO2 Growth Kinetics Models: Deal-Grove Model A & B/A B = C1e − E1 B = C2 e A kbT − E2 k bT (oxidant diffusion) (interface reaction rate) Plots of B & B/A using values in table. Knowlton 15 MSE 310 Elec. Props of Matls Knowlton Thermally Activated Processes - Arrhenius Behavior - Chemical Vapor Deposition (CVD) J1 J2 J SBL = J1 J rxn = J 2 = k s Cs = − Dgas = Dgas δS ∂C ∂x (C gas − Cwaf. surf. ) = hg ( C gas − Cwaf. surf. ) Knowlton 16 8