Elementary Materials Science Concepts Atomic Structure:
advertisement
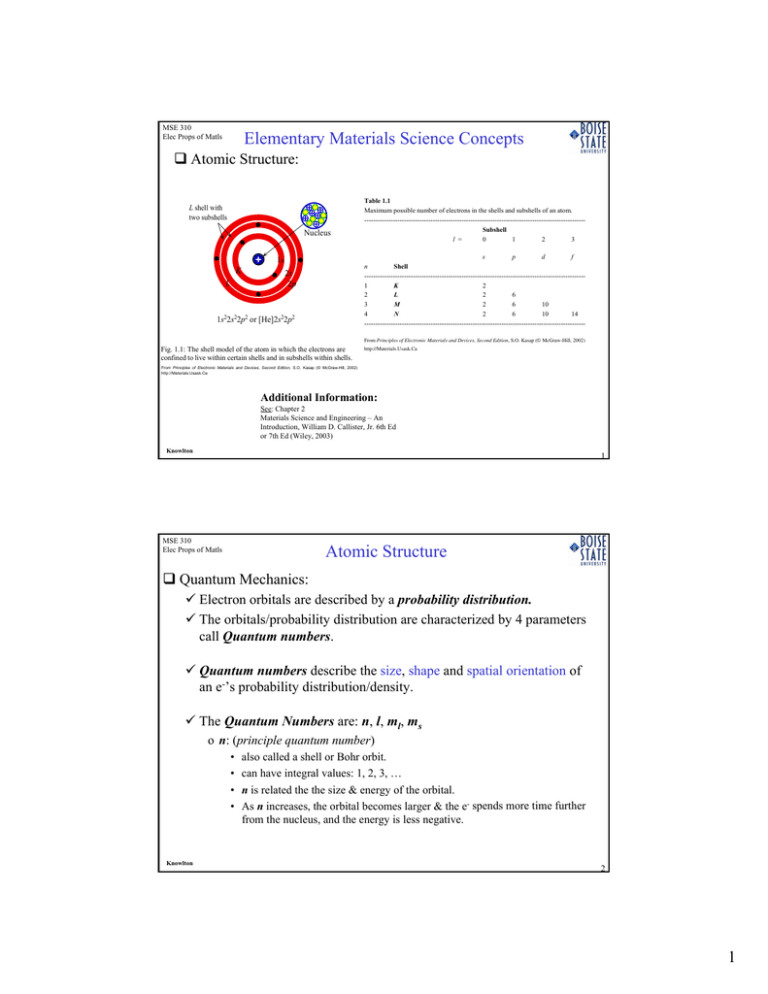
MSE 310 Elec Props of Matls Elementary Materials Science Concepts Atomic Structure: L shell with two subshells Nucleus s p d f n Shell ---------------------------------------------------------------------------------------------------1 K 2 2 L 2 6 3 M 2 6 10 4 N 2 6 10 14 ---------------------------------------------------------------------------------------------------- 1s K L Table 1.1 Maximum possible number of electrons in the shells and subshells of an atom. ---------------------------------------------------------------------------------------------------Subshell l = 0 1 2 3 2s 2p 1s22s22p2 or [He]2s22p2 From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002) Fig. 1.1: The shell model of the atom in which the electrons are confined to live within certain shells and in subshells within shells. http://Materials.Usask.Ca From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002) http://Materials.Usask.Ca Additional Information: See: Chapter 2 Materials Science and Engineering – An Introduction, William D. Callister, Jr. 6th Ed or 7th Ed (Wiley, 2003) Knowlton 1 MSE 310 Elec Props of Matls Atomic Structure Quantum Mechanics: 9 Electron orbitals are described by a probability distribution. 9 The orbitals/probability distribution are characterized by 4 parameters call Quantum numbers. 9 Quantum numbers describe the size, shape and spatial orientation of an e-’s probability distribution/density. 9 The Quantum Numbers are: n, l, ml, ms o n: (principle quantum number) • • • • Knowlton also called a shell or Bohr orbit. can have integral values: 1, 2, 3, … n is related the the size & energy of the orbital. As n increases, the orbital becomes larger & the e- spends more time further from the nucleus, and the energy is less negative. 2 1 MSE 310 Elec Props of Matls Atomic Structure Quantum Mechanics: 9 The Quantum Numbers are: n, l, ml, ms o l: (azimuthal quantum number) • Also called a subshell (of the shell, n) • can have integral values from 0 to n-1 for each value of n. » l = 0, 1, 2, …, (n-1) (known as a selection rule) • This quantum number relates to the shape of atomic orbital. • The value of l for a particular orbital is commonly assigned a letter: » l=0 is called s; l=1 is called p; l=2 is called d; l=3 is called f o ml: (magnetic quantum number) • Can have integral values between l & –l, including zero. » m = -l, …, -2, -1, 0, +1, +2, …, +l (known as a selection rule) • The value of ml relates to the orientation of the orbital in space relative to the other orbitals in the atom. Knowlton MSE 310 Elec Props of Matls 3 Atomic Structure Quantum Mechanics: 9 The Quantum Numbers are: n, l, ml, ms Knowlton S. Zumdahl, Chemistry (Heath, 1986) 4 2 MSE 310 Elec Props of Matls Atomic Structure Quantum Mechanics: 9 The Quantum Numbers are: n, l, ml, ms o ms: (electron spin magnetic number) • Can have the value 1/2 or -1/2 » ms = ±½ (selection rule) • Explains the electron's magnetic moment with two possible orientation • Often described as "spin up" (1/2) or spin down (-1/2) 9 What is the consequence or result of ms? • In a given atom, no two e-'s can have the same set of four quantum numbers. • An orbital defined by n, l, mj, can hold only two e-'s, and they must have opposite spins. PauliExclusion Exclusion Pauli Principle: Principle: Knowlton 5 MSE 310 Elec Props of Matls Atomic Structure Quantum Mechanics: ml ms ml = m ms = s/h Eqn. 2-46 refers to selection rules Knowlton Streetman & Banerjee, Solid State Electronic Devices (Prentice Hall, 2000) 6 3 MSE 310 Elec Props of Matls Atomic Structure Quantum Mechanics: 9 From the 1st 2 Quantum Numbers, n and l, we can determine the electronic configuration of atoms which is logically followed by the periodic table – how e-’s fill sub-orbitals. 9 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, … 9 1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s2, 4p6, 4d10, … 9 H: 1s1 o o o o o o o o o He: 1s2 Li: 1s2, 2s1 Be: 1s2, 2s2 B: 1s2, 2s2, 2p1 C: 1s2, 2s2, 2p2 N: 1s2, 2s2, 2p3 O: 1s2, 2s2, 2p4 Cl: 1s2, 2s2, 2p5 Ne: 1s2, 2s2, 2p6 SubOrbital Value of l quantum number Number of states s 0 2 p 1 6 d 2 10 Knowlton 7 MSE 310 Elec Props of Matls Atomic Structure halogens noble gases alkaline earth metals alkali metals Atomic Structure – Periodic Table transition metals 103 Lr Knowlton 104 Rf 105 Db 106 Sg 107 Bh 108 Hs 109 Mt 8 4 MSE 310 Elec Props of Matls Atomic Structure Atomic Structure – Periodic Table 9 Electronic configuration Knowlton MSE 310 Elec Props of Matls S. Zumdahl, Chemistry (Heath, 1986) 9 Atomic Structure Atomic Structure – Periodic Table 9 Electronic configuration Knowlton S. Zumdahl, Chemistry (Heath, 1986) 10 5 MSE 310 Elec Props of Matls Atomic Structure Atomic Structure – Periodic Table 9 Electronic configuration S. Zumdahl, Chemistry (Heath, 1986) Knowlton 11 MSE 310 Elec Props of Matls Atomic Structure Atomic Structure – Periodic Table 9 Electronic configuration: o Aufbau Principle: Just as protons are added one by one to the nucleus to build up the elements, electrons are similarly added to these hydrogen like orbitals. Element Elec. Conf. 1s 1 H: He: 1s 2 Li: 1s 22s 1 Be: 1s 22s 2 B: 1s 22s 22p1 C: 1s 22s 12p2 N: 1s 22s 12p3 O: 1s 22s 12p4 F: 1s 22s 12p5 1s 2s ↑ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ 2p ↑ ↑ ↑ ↑↓ ↑↓ ↑ ↑ ↑ ↑↓ ↑ ↑ ↑ o Hund’s Rule: the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli Exclusion Principle in a particualr set of degenerate orbitals. Knowlton o Degenerate orbitals: orbitals that have the same energy (same n, l, ml). 12 6 MSE 310 Elec Props of Matls Atomic Structure Atomic Structure – Periodic Table & Periodic Trends 9 Atomic Radius S. Zumdahl, Chemistry (Heath, 1986) Knowlton 13 7