Document 10529038
advertisement
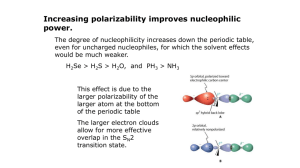
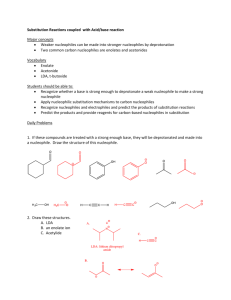
Chem 634 Spring 2015 Nucleophilic Substitution Chemistry Announcements Organic Journal Club: Thursdays, 12:30–1:30, 219 BRL Contact: Corey Basch (cbasch@udel.edu) organizes the OJC website and schedule. Today a) alcohol activation b) substitutions at alkyl centers c) ester formation d) amide formation Reading: C&S 3.1-3.2, 3.4 Alkyl Nucleophilic Substitution Be sure you understand SN1, SN2 and elimination mechanisms. Common Problem: OH Me Me Nuc Nuc Nuc Me Me OH (poor leaving group) Need to activate ROH Sulfonates O O OH Me O S Cl O R Me Me "py" N py Me O O Cl H O S R Me O S Me R note retention Sulfonates common sulfonates O S O O S Me O O S CF3 O Me toluenesulfonate "tosylate" "Ts" common reagents O Cl S O Me (TsCl) methanesulfonate "mesylate" "Ms" trifluoromethanesulfonate "trifate" "Tf" MsCl O O F3C S O S CF3 O O (Tf2O) Note: alkyl triflates are normally unstable Sulfonates Recall: R1 R1 Nuc LG Nuc R2 H R1 LG Nuc H R2 R2 H σ*C-LG transition state OSO2Me Me Me Nuc Nuc SN2 Me Me LG Sulfonates O O S OH Me O Cl O R Me Me O S Me R retention "py" N OSO2Me Me Me Nuc SN2 Me Nuc overall inversion! Me inversion Alkyl Halides Formation of alkyl chlorides: OH Me Cl Me O S Cl Cl CH2Cl2, py O Me O S Cl Me Cl pyH+ thionyl chloride Me Me + SO2, pyHCl Alkyl Halides Cl Me Nuc Nuc Me Me Me note: overall rentention compared to R–OH Alkyl Halides Formation of alkyl bromides: PPh3, Br2 R OH R Br Alkyl Halides Formation of alkyl bromides: Mechanism: Ph3P Br Br Ph3P Br Br ROH Br R H O R PPh3 O - HBr PPh3 - Ph3P O Br Ph3P O Ph3P O triphenylphosphine oxide very thermodynamically favorable R Br Alkyl Halides Similar reactions for ROH to RX: X = Br X = Cl PBr3 PCl3 O PPh3 + CCl4 PPh3 + N Br N-Bromosuccinimide "NBS" O X=I PPh3 + I2 + imidazole N N H You need to be able to draw the mechanisms for these reactions. The Finkelstein Reaction R Br + NaI R I + NaBr not soluble in acetone Pg 170 in Kurti & Czako Reactivity of Alkyl Electrophiles For RX TfO>>>>>TsO > I > MsO > Br >> Cl unstable sluggish Most Often Used RX in SN2-type Reactions Br Me-I benzyl bromide "BnBr" Cl allyl chloride WHY? Small or activated. No beta-hydrogen atoms. NO E2! Nuc H Me Me X How to Control SN2 vs E2 Me H Me X Nuc vs Me Nuc SN2 E2 small nucleophiles “non-basic” nucleophiles methyl, 1° or 2° substrates large nucleophiles “basic” nucleophiles 3° substrates (SN2 very rare) Note: In most cases, SN2 vs E2 compete. “Basic” vs “Non-Basic” MUST KNOW pKa TABLE (every organic chemist should know these) Note water and DMSO numbers do not match. Why? For SN2 vs E2, cutoff is ~ pKa = 12-16 Also see: Evans pKa Table (Harvard) Reich pKa Table (UW Madison) http://www2.lsdiv.harvard.edu/labs/evans/pdf/evans_pKa_table.pdf http://www.chem.wisc.edu/areas/reich/pkatable/index.htm Using pKa to Predict Equilibrium Consider: Treat As: MeOLi + Bu-H MeOH + BuLi MeOH + Bu- Karxn =? MeO- + Bu-H Ignores counter-ion. Not a prefect assumption. In some cases, down right bad, but it gives us a start. Definitions: BuH + H2O MeO- MeOH + H2O Ka2 Ka1 = 10-50 Bu- + H3O+ = + H3 [MeO-][H3O+] [MeOH][H2O] O+ pka1 = 50 Ka2 = 10-17 pka2 = 17 =10-17 Using pKa to Predict Equilibrium Bu- + H3O+ BuH + H2O BuH + H2O rewrite: Bu- + H3O+ Ka3 = [BuH][H2O] [Bu-][H3O+] Ka3 = 1/Ka1 =1/10-50 =1/10-50 Using pKa to Predict Equilibrium MeOH + Bu- [MeO-][BuH] Karxn = = MeO- + Bu-H [Bu-][MeOH] [MeO-][H3O+] [MeOH][H2O] · = Ka2 • Ka3 [BuH][H2O] [Bu-][H3O+] = 10-17 • 1/10-50 = 10(-17-(-50)) = 1033 Note: pKa(Bu–H) = 50 pKa(MeO–H) = 17 Alkylation With Carbon Nucleophiles R CN Br Na+, –CN K+ R CN pKa 9.4 (12.9) Note: C–C bond formed We will see many more carbon nucleophiles later in the class. Oxygen Nucleophiles "Williamson Ether Synthesis" R OH pKa (30) MeI, NaH pKa' ~36 R OMe For 1° & 2° RX, E2 can compete OH Ph Br O K2CO3 pKa 10 (18) O Typically solvent is DMF or DMSO H NMe2 Me O S Me Oxygen Nucleophiles O R O BnBr OH Cs2CO3 DMF R OBn Mitsunobu Reaction DEAD Me Me OH Me Me PPh3, RCO2H R O O O DEAD = EtO O N N OEt Upsides: • works with many acidic nucleophiles • clean SN2 chemistry • often works when other reactions won't Downsides: • DEAD not overly stable • waste Mitsunobu, Synthesis, 1981,1, 1 Mitsunobu Reaction Mechanism: O O O O N N EtO OEt EtO N N PPh3 O O PPh3 O EtO O N N H PPh3 N N H EtO OEt O R OEt OEt PPh3 H O OH R' H O R" R' R" O O EtO O N N H H waste OEt R' R O O PPh3 + O R" O R' R R" + OPPh3 waste Nitrogen Nucleophiles Challenge: Overalkylation… NH2 Me–I N H Me + Me N Me + Me Me N Me I Nitrogen Nucleophiles Classical: Gabriel Synthesis O NK O + R R Br + KBr N O O O H2N NH2 R NH2 + 2 steps HN HN O Nitrogen Nucleophiles Azides Me Me Br NaN3 pKa' 7.9 Me Me [Red] Me N3 Caution!: • NaN3 ~1000x more toxic than NaCN • Azides can explode Me NH2 Nitrogen Nucleophiles Amides soft O R O MeI NHEt Cs2CO3 DMF O MeO S OMe O dimethyl sulfate LiAlH4 N Et Me R hard OMe R NMe "imidate" R N Et Me