GEOS 470R/570R Volcanology L03, 23 January 2015 Handing out
advertisement

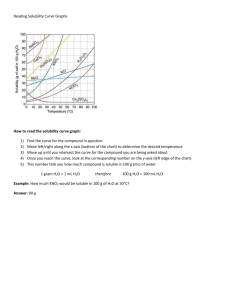
GEOS 470R/570R Volcanology L03, 23 January 2015 Handing out Summary of slides Hildreth (1981) (for L04) Metz and Mahood, 1985 (for L05) McPhie et al., 1993, p. 21-33, 66-71 As preparation for Lab 1 (Monday), read p. 21-33 and 66-69 “If we are facing in the right direction, all we have to do is keep on walking.” --Buddhist Proverb Readings from textbook For L03 from Lockwood and Hazlett (2010) Volcanoes—Global Perspectives Chapter 3 For L04 from Lockwood and Hazlett (2010) Volcanoes—Global Perspectives Chapters 2 and 3 Assigned reading For today L03 None For L04, 26 January 2015 Hildreth, W., 1981, Gradients in silicic magma chambers: Implications for lithospheric magmatism: Journal of Geophysical Research, v. 86, p. 10,15310,192. For L05, 30 January 2015 Metz, J. M., and Mahood, G. A., 1985, Precursors to the Bishop Tuff eruption: Glass Mountain, Long Valley, California: Journal of Geophysical Research, v. 90, p. 11,121-11,126. Study this diagram frequently for the lab and before first field trip McPhie et al., 1993, Fig. 33 Photographic context for the laboratory exercise: Aerial view from the resurgent dome toward Glass Mountain on the NE rim of the Long Valley caldera Glass Mountain http://lvo.wr.usgs.gov/gallery/30714277-095_caption.html Photo by C. D. Miller, 1982 http://lvo.wr.usgs.gov/gallery/GalleryMap.html Photographic context for the laboratory exercise: Pine Grove, UT Photo E. Seedorff, May 1979 Superstition Mountains Last time: Physical and chemical properties of magmas Time, length, area, volume, and energy scales Chemical and mineralogical characterization of volcanic rocks Physical properties Temperature T° Viscosity η Density ρ Thermal conductivity k Crystallization rates Compositions: Silica content Ultramafic IUGS divisions commonly followed for ultramafic to andesite No agreement on terms for silicic rocks <45 wt% SiO2 Basalt 45 – 52% Basaltic andesite 52 – 57% Andesite 57 – 63% Dacite 63 – 68% Rhyodacite (quartz latite) 68 – 72% Rhyolite 72 – 75% High-silica rhyolite 75 – 77.5% IUGS has only two terms for SiO2 > 63 wt% (dacite and rhyolite) Many people who work on non-alkalic silicic rocks use a subdivision similar to what is at left Eruptive volumes DRE = dense-rock equivalent Vdre ≈ 0.6 V for tephra Vdre ≈ V for lavas Volumes (DRE) for eruptions of the last century Katmai-Novarupta, AK Pinatubo, Philippines Mount St. Helens, WA June 1912 June 1991 May 1980 13 km3 5 km3 0.5 km3 Comparison Huckleberry Ridge, Yellowstone 2.0 Ma Bishop Tuff, Long Valley, CA 0.7 Ma 2500 500 km3 km3 Hildreth, 1981; Wohletz and Heiken, 1992; Wolfe and Hoblitt, 1996 Temperature summary Composition Temperature (°C) Rhyolite-rhyodacite 700-900 Dacite 800-1100 Andesite 950-1170 Mafic (tholeiites) 1050-1250 Alkali basalts and nephelinites Ultramafic (komatiites) 900-1100 1400-1700 (est.) Williams and McBirney, 1979, Table 2-2; Cas and Wright, 1987, Table 2.3; Kilburn, 2000, Table 2 Viscosity η Melt viscosity issues Temperature [η ↓ with ↑ T] Dissolved volatile content, especially water content [η ↓ with ↑ H2O] Chemical composition, especially silica content [η ↑ with ↑ SiO2] Crystal content [η ↑ with ↑ volume fraction solids] Viscosity: Network formers and Network modifiers Network formers contribute to η ↑ Network modifiers contribute to η ↓ Si, Al Network formers (strong bonds with O) (η ↑) Fe, Mg, Ti, others Network modifiers (η ↓) Alkalis: Na, K, Rb, Cs Network formers in peraluminous and metaluminous melts (η ↑) Network modifiers in peralkaline rocks (η ↓) Volatiles: H2O, F, Cl Network modifiers (η ↓) Viscosity comparison e.g., Hawaiian tholeiite 1200°C 1130°C By comparison, H2O 25°C η = 500 poise = 50 Pa s η = 8000 poise = 800 Pa s η = 0.01 poise = 0.001 Pa s If basalts are much more viscous than water, why, then, do basalts flow fairly rapidly? Viscosity changes during flow Typically increases by 2 to 10X from vent to toe of flow Primarily because of loss of volatiles Minor effect of cooling Density summary (at liquidus temperature and anhydrous, except as noted) Composition Granite / rhyolite Granite / rhyolite (2 wt% H2O) Granodiorite / dacite Gabbro / basalt Komatiite Liquidus Density Density T° (°C) (kg/m3) (g/cm3) 900 2349 2.35 900 2262 2.26 1100 2344 2.34 1200 1500 2591 2748 2.59 2.75 Spera, 2000, Table 3 Importance of density Important control on rise of magmas through crust Strong control on fluid dynamics of magmas Petrologic implications for mixing of magmas Transport of magmatic heat Convection Heat transported by bulk flow Conduction (phonon conduction) Phonon = quantized thermal waves Heat transported by atomic vibration of lattice Radiation Electromagnetic phenomenon involving photon transfer Crystallization rates Rate decreases as viscosity increases Rate ↓ with ↑ η Consequences Rhyolites (high η) crystallize slowly glassy groundmass Basalts (low η) crystallize rapidly fine crystalline groundmass Recrystallization of glass Rhyolitic glass silica mineral + alkali feldspar (and/or clay minerals and zeolites in alkaline lakes) Hydrate and crack Nucleate crystals along cracks Summary: Chem & Phys Properties The time, length, area, volume, and energy scales of volcanism and volcanic rocks Each vary by many orders of magnitude, but Characteristic features vary within fairly narrow ranges Mineralogy is a function of chemical composition Silica content and alkalinity are key compositional variables The most important physical properties are Temperature T°, Viscosity η, Density ρ, Thermal conductivity k, and Crystallization rates Impacts on viscosity η ↓ with ↑ T; η ↓ with ↑ H2O and most other volatiles; η ↑ with ↑ SiO2; η ↑ with ↑ volume fraction solids (e.g., phenocrysts) The properties are not independent of one another Many can be linked to chemical composition of the magma Many observations can be explained in terms of viscosity (e.g., shapes of volcanoes, eruptive style) Lecture 03: Volatiles and sampling magmatic gases Volatiles Undersaturation vs. saturation Solubility (saturation limit) Solubility controls H2O CO2 S Halogens: Cl, F Volatiles How do we know that volatiles (e.g., H2O or F) are important constituents of magmas on Earth? Volatile An element (e.g., S) or compound (e.g., H2O or CO2) that forms a gas at relatively low pressure and magmatic temperature Can be dissolved in silicate melts Can occur as bubbles of exsolved gas Can be incorporated in the structure of phenocrysts Why are volatiles important? Volatiles modify the behavior of silicate melts and magmas (last lecture) e.g., decrease viscosity and density As magmas ascend toward the surface, decrease in pressure may cause exsolution of volatiles Form bubbles Could cause great increase in volume Hence, volatiles are widely thought to be important in governing eruptive phenomena Important ligands to transport metals to form certain types of ore deposits Bursting gas bubbles in lava lake at Kilauea, Hawaii Schmincke, 2004, Fig. 4.30 Important magmatic volatiles Water (H2O) Carbon dioxide (CO2) Sulfur (SO2, H2S) Halogens (Cl, F) Other volatiles (He, Ar, B) are minor components Nonetheless, my provide clues to sources of magmas and origin of atmosphere Differences in proportions of volatiles as a function of magma composition Basaltic CO2 H2O Rhyolitic H2O SO2 F Cl Schmincke, 2004, Fig. 4.18 CO2 SO2 Solubility Maximum amount of a species or component that can be dissolved under a given set of conditions P T° X (melt composition) If melt contains less than maximum amount possible at given conditions, then the melt is undersaturated with respect to that volatile component Magmas are not necessarily volatile-saturated, although many may be saturated prior to eruption Solution mechanisms for H2O in silicate melts Speciation controlled by amount dissolved At low concentrations of H2O Dissolves by forming OH- groups that are structurally bound to the aluminosilicate network Indicated because solubility varied by P(H2O)0.5 Concentration via this mechanism varies directly with T As total dissolved H2O increases, Relative proportion of molecular H2O increases Concentration via this mechanism independent of T Proportions: OH- groups = molecular H2O At 3 wt% dissolved H2O for rhyolites At 3.5 wt% dissolved H2O for basalts Solution mechanisms for H2O as a function of dissolved water content Best and Christiansen, 2001, Fig. 4.9; after Silver et al., 1990 Dissolution of H2O forming OHgroups Dissolved oxygen forming hydroxyl ions Breaking O-Si-O polymers Reducing degree of polymerization of melt Predicted effect on viscosity? Best and Christiansen, 2001, Fig. 4.8 Recall: Viscosity η Melt viscosity issues Temperature [η ↓ with ↑ T] Dissolved volatile content, especially water content [η ↓ with ↑ H2O] Chemical composition, especially silica content [η ↑ with ↑ SiO2] Crystal content [η ↑ with ↑ volume fraction solids] Solubility of H2O Strongly dependent on pressure [↑ solubility with ↑P) Gaseous form has larger partial molar volume than does water when dissolved in silicate melt Typical for many other volatiles Dependent on composition [↑ solubility with ↑ SiO2] Wallace and Anderson, 2000, Fig. 1 Degassing of Bocca Nova crater, summit of Mount Etna, Italy Hot H2O gas is condensed to water At the boundary between the rising gas stream and The cold atmosphere at the summit of the volcano Schmincke, 2004, Fig. 4.20 Expansion of H2O 17,000 X expansion from liquid to steam at sea level Drives phreatic eruptions (steam explosions) These were the early eruptions in early 1980 at Mount St. Helens (pre-climactic eruption) Why would they occur early? Concentration of water as a function of magma composition Schmincke, 2004, Table 4.1, after Fisher and Schmincke, 1984 Solubility of CO2 CO2 is less soluble in magmas than H2O By 1 to 2 orders of magnitude by weight at same P, T° Solubility of CO2 greater in rhyolite than basalt As with H2O Speciation of dissolved CO2 varies according to bulk melt composition Rather than according to volatile concentration, as for H2O Solubility of CO2 Bulk melt compositional effect for solution mechanism CO2 dissolves in rhyolitic melt as CO2 molecules CO2 dissolves in basaltic melt as a dissolved carbonate ion Intermediate compositions have both species Wallace and Anderson, 2000, Fig. 2 Solution mechanisms for CO2 in silicate melts Varies according to bulk silicate melt composition Silica-poor melts (basalts, basanites, nephelinites) As structurally bound CO32 Solubility of CO2 as carbonate increases with decreasing silica content Silica-rich melts (rhyolites) CO2 molecules Intermediate silica compositions As structurally bound CO32- and as CO2 molecules CO2 bubbles in lake at Laacher See, Eifel, Germany (erupted 12 900 yr B.P.) Schmincke, 2004, Fig. 4.22 Multicomponent systems Likely to have numerous volatile species present If sum of partial pressures of all dissolved species exceeds confining pressure, magma will be saturated with a multicomponent gas phase Gas phase will not be “pure” Because of different solubilities, elements will partition differently between melt and vapor H2O and CO2 At saturation and constant total pressure, increase in partial pressure of P(H2O), and hence dissolved H2O in the melt, will depress P(CO2) and dissolved CO2 Hence negative slopes Wallace and Anderson, 2000, Fig. 3 Sulfur—Complex behavior Occurs in multiple oxidation (valence) states Reduced form: sulfide S2- (species HS-, S2-) Oxidized form: sulfate S6+(species SO42-, HSO4-) Maximum S that can be dissolved in silicate melt is controlled by saturation of the melt with a sulfur-bearing phase (liquid or solid) Sulfur-bearing phases that control S content of silicate melt Immiscible Fe-rich sulfide liquid (with ~10% O) In high-temperature basaltic magmas at relatively low f(O2) Crystalline pyrrhotite (Fe1-xS) Common in andesitic to rhyolitic magmas Pyrrhotite may coexist with a Cu-Fe-sulfide mineral, intermediate solid solution (iss) In andesitic to rhyolitic magmas At higher f(O2), may crystallize anhydrite First reported as a microphenocryst in trachyandesite tephra from El Chichón, México Microphenocrysts dissolve in rain water Dissolved sulfur At relatively low oxidation states, sulfide is the dominant form of dissolved sulfur At relatively high f(O2), sulfate is the dominant form of dissolved sulfur Within a few log units f(O2) of NNO buffer, both dissolved sulfide and sulfate significant Wallace and Anderson, 2000, Fig. 4 Sulfur solubility S more soluble at high oxygen fugacities Where dissolved sulfur occurs as sulfate Anhydrite present Wallace and Anderson, 2000, Fig. 5 Sulfur solubility The solubility of reduced S (sulfide, S2-) increases with Fe concentration in the melt Wallace and Anderson, 2000, Fig. 6 Sulfur solubility Important temperature effect Solubility of sulfur increases with increasing temperature Under both oxidizing and reducing conditions Wallace and Anderson, 2000, Fig. 5 Degassing of S-rich gases from fumaroles at Vulcano, Italy Schmincke, 2004, Fig. 4.23 Degassing of bluish S-rich gases from open central conduit of crater at Masaya, Nicaragua Schmincke, 2004, Fig. 4.24 Degassing of CO2 and SO2 from Mount Etna, Italy—Why different abundances? Schmincke, 2004, Fig. 4.21, after Allard et al., 1991 Solubility controls for Cl Cl solubility strongly dependent on silicate melt composition Solubility increases as ratio of (Na + K) / Al in the silicate melt increases Maximum Cl content of melt is when melt is saturated with immiscible alkali chloride melt (molten salt) Saturation concentration varies with P, T°, dissolved H2O, silicate melt composition Solubility of Cl For silicate melts saturated with both H2O and CO2 Maximum Cl solubilities range from X000 ppm to ~2 wt% Cl Cl partitions strongly into vapor phase Concentrations in vapor can be 5-20 X mass dissolved in melt Hence, solubility of Cl is intermediate between H2O and CO2 Possible for melt to be saturated with a hydrosaline melt, H2O, and CO2 Solubility of F Solubility strongly dependent on silicate melt composition Highly soluble in silicate melts (to 10 wt % F)—similar to H2O Natural melts generally have much lower dissolved F Sn-W rhyolitic magmas as much as 5 wt% F Ultrapotassic mantle-derived magmas as much as 2 wt% F Summary Volatile: Element or compound that forms a gas at low P and T° Typically a multicomponent system in nature (mixed volatiles) Conditions: Undersaturated vs. saturated Solubility: Saturation limit Solution mechanisms differ widely; can be a function of Amount of volatile dissolved (H2O) Bulk silicate melt composition (CO2, S, Cl, F) Oxidation state / oxygen fugacity (S) Solubility of pure volatiles is a function of many factors Pressure (H2O) Bulk silicate melt composition, including other volatiles (H2O, CO2, S) Oxidation state / oxygen fugacity (S) Presence of separate sulfide phase (S) Next time: Petrologic overview