Document 10449348
advertisement

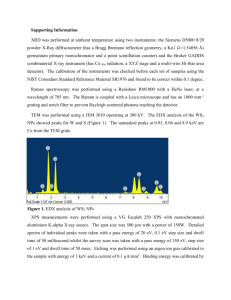
Laser is acronym for light amplification by stimulated emission of radiation • Most lasers are made of some material such as a crystal of ruby or a gas that is enclosed in a glass tube, like argon. • Suppose an atom was in an excited state. I.e. an electron was in a higher energy orbit. If a photon of light, that has the same energy as the difference between the excited state and the ground state, passes by the atom then it might cause the excited electron to drop to its ground state and emit another photon of the same wavelength. This is the stimulated emissions part of the definition. • Furthermore, the second photon is exactly in phase with the first photon. When many photons are in phase, we say that the light is coherent. • Suppose that the ruby crystal or the gas is constantly being excited. Then if a first photon is created, and this would happen spontaneously, then it will create a second one that is coherent. • As the photons travel along the tube or crystal, they create more photons, all of which are coherent. Therefore, by constructive interference the light quickly becomes bright. The crystal or the gas is constantly being excited so after the photon is emitted, the atoms quickly get re-excited. • The excited atoms are enclosed with mirrors at both ends. This way the photons hit the mirror and are bounced back to pass through the material repeatedly. Only the photons that are travelling precisely along the axis of the tube will remain, as the others will bounce right out of the sides of the tube. Thus a beam of very bright, coherent light is created that is aligned precisely through a cascading effect. The photons will bounce back and forth millions of times. • The mirrors are not perfect. They let out about 5% of the light. The light that escapes forms the laser beam. Two common lasers used for Raman spectroscopy are: 1) Gas laser (eg. Argon, above) 2) Solid state laser (Nd:YVO4 left) Sample Holographic notch filter, to remove laser line Examples of Raman Instruments Spectrum is defined by: 1) x-axis = position of peaks 2) y-axis = intensity of peaks x-axis represents an energy shift = c/, where = frequency, c = speed of light, = wavelength Raman shift = ύ = /c = 1/ (cm-1) But E = h = hc/ = hcύ, and since hc is a constant, then Raman shift is proportional to change in energy Origin of the Energy shift •Consider the harmonic oscillator, with restoring force proportional to displacement: F = -kx, F = force, k = force constant, x = displacement •Then x(t) = Asin(2t + ), A is amplitude, t = time, = phase shift •Solve for F = ma = -kx to obtain = 1/2 sqrt(k/m), ie. Frequency depends on mass and strength of bonds •Energy is then E = h (n+1/2), where n = vibrational quantum number Vibrational modes for BF3 Weak bonds Heavy elements Strong bonds Light elements Raman Frequency vs. M-O Bond Length 1700 NO3 1600 CO3 Raman Frequency (cm-1) 1500 IIIBO3 IVBO4 1400 SO4 PO4 1300 SiO4 1200 BeO4 CrO4 1100 AsO4 VO4 1000 MoO4 WO4 900 800 700 600 1.2 1.3 1.4 1.5 1.6 Bond Length (Å) 1.7 1.8 1.9 How do you compute the positions of the peaks? •Lattice dynamics •F = -kx •E = ½ mv2 = kinetic energy • ½ xt D x, where D = dynamical matrix •Eigenvalues provide frequencies, eigenvectors provide normal modes, some can be the same as others, known as degenerate modes How many peaks are there? •In general, there are 3n-5 modes, where n = number of atoms in unit cell, and the 5 represents translation and rotation of unit cell •Bilboa crystallographic server, http://www.cryst.ehu.es/ •Using crystal structure information, you can determine the number of peaks How does chemistry affect the positions? •C = XP, where C = matrix of chemistry X = transformation matrix P = peak position matrix •Then CPt = XPPt X = CPt(PPt)-1 •50 garnets, X3Y2(SiO4)3, •16 elements, average error is 0.02 atoms per chemical formula Raman spectra of garnets Effect of pressure pyroxene Effects of temperature Soft modes Artifacts that should be removed from a spectrum 1. Cosmic rays 2. Notch filter effects