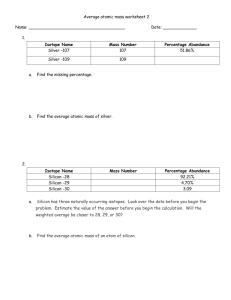
Sacrificial High-Temperature Phosphorus Diffusion Gettering
advertisement

Sacrificial High-Temperature Phosphorus Diffusion Gettering for Lifetime Improvement of Multicrystalline Silicon Wafers by MASSACHUSETTS INSTITUTE OF TEC-NOLOGY Stephanie Morgan Scott AUG 15 2014 B.S. in Mechanical Engineering University of California, Berkeley (2012) LIBRARIES ARCHN ES Submitted to the Department of Mechanical Engineering in partial fulfillment of the requirements for the degree of Master of Science in Mechanical Engineering at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2014 2014 Massachusetts Institute of Technology. All rights reserved. Author....................................... Signature redacted Department of Mechanical Engineering May 9, 2014 Signature redacted ....................... Tonio Buonassisi Assistant Professor of Mechanical Engineering Thesis Supervisor C ertified by ........................................ ,................ Signature redacted Accepted by................... ..................... .. David E. Hardt Professor of Mechanical Engineering Chairman, Department Committee on Graduate Theses Sacrificial High-Temperature Phosphorus Diffusion Gettering for Lifetime Improvement of Multicrystalline Silicon Wafers by Stephanie Morgan Scott Submitted to the Department of Mechanical Engineering on May 9, 2014 in Partial Fulfillment of the Requirements for the Degree of Master of Science in Mechanical Engineering ABSTRACT Iron is among the most deleterious lifetime-limiting impurities in crystalline silicon solar cells. In as-grown material, iron is present in precipitates and in point defects. To achieve conversion efficiencies in excess of 20%, bulk minority-carrier lifetimes in excess of 300 Rs (p-type) are required [1]. For cost-effective multicrystalline silicon wafers, achieving this lifetime often requires gettering. Gettering at higher temperatures for longer times is often necessary to fully dissolve and remove precipitated impurities. However, such time temperature profiles can result in unacceptably deep emitters, affecting the blue response of the finished device. Here, we explore a "sacrificial" gettering step, in which gettering and emitter-formation steps are decoupled and optimized independently. The optimization of the sacrificial gettering step is guided by the Impurity-to-Efficiency simulation tool [2] and explores higher temperatures (up to 1100*C) than standard industrial processes (typically 820-850*C). The models indicate that iron concentration should be reduced by higher-temperature gettering, which is confirmed by experiment. However, uniform lifetime degradation occurs at higher temperatures, suggesting another homogeneously distributed defect is generated as a result of the high-temperature gettering process. A list of candidate defects is presented. Thesis Supervisor: Tonio Buonassisi Title: Professor of Mechanical Engineering 3 4 ACKNOWLEDGEMENTS Thank you to the numerous people and groups that have supported me throughout my research at MIT. Thank you to Professor Buonassisi for the opportunity to research in the MIT PV Lab. Your contagious enthusiasm research and future of energy sparked my curiosity. Thank you to all my colleagues in the PV Lab. Special thanks to Jasmin Hofstetter for providing invaluable support including teaching me experimental techniques and sharing knowledge from your tremendous background in photovoltaics. Thank you to David Fenning for your instrumental introduction to gettering. Also, thank you to my fellow team silicon members David Berney Needleman, Ashley Morishige, Doug Powell, Sergio Castellanos and Mallory Jensen. Thank you to Tim Kirkpatrick and Jungwoo Lee my "solar boot camp" drill sergeants who trained me to fabricate a solar cell. Thank you to all the students that I have been privileged to work with while at MIT. Everyone's commitment to his or her work is a constant source of inspiration. A special thanks is due to the students in my office, 35-135, for their constant liveliness, ingenuity, and support. Thank you to friends and family for endless encouragement. My research was financially supported by the MIT Department of Mechanical Engineering's Rohsenow Fellowship and the Quantum Energy and Sustainable Solar Technologies (QESST) an Engineering Research Center (ERC) sponsored by the National Science Foundation (NSF) and the U.S. Department of Energy (DOE). 5 6 CONTENTS A bstract.......................................................................................................................................... 3 A cknow ledgem ents ....................................................................................................................... 5 Contents ......................................................................................................................................... 7 Figures............................................................................................................................................ 9 1 Photovoltaic Energy............................................................................................................... 13 1.1 Introduction...................................................................................................................... 13 1.2 Photovoltaic Basics....................................................................................................... 13 2 Iron in Silicon ........................................................................................................................... 17 2.1 Silicon PV M aterial....................................................................................................... 17 2.2 Silicon Material Purity .................................................................................................. 17 2.3 Silicon Manufacturing Process..................................................................................... 18 2.4 Iron States in Silicon.................................................................................................... 19 2.5 G ettering .......................................................................................................................... 19 2.6 D esigning a Gettering Profile ..................................................................................... 22 3 Simulated Iron Response to Gettering Time-Temperature Profile ................................. 23 3.1 M otivation for Sim ulation........................................................................................... 23 3.2 M odel of Iron in Silicon................................................................................................ 23 3.3 M inority Carrier Lifetim e ............................................................................................ 26 3.4 Sim ulation of H igh-Tem perature Gettering.................................................................. 27 3.5 Transient Response ...................................................................................................... 28 3.6 Other Im purities in Silicon........................................................................................... 31 4 Experimental Methods for High-Temperature Phosphorus Diffusion Gettering .......... 33 4.1 Introduction...................................................................................................................... 33 4.2 M ulticrystalline Silicon Sam ples ................................................................................ 33 7 4.3 Sample Preparation - Saw Damage Etch..................................................................... 34 4.4 M inority Carrier Lifetime M easurem ent .................................................................... 35 4.5 Interstitial Iron Quantification by FeB Dissociation................................................... 36 4.6 Phosphorus Diffusion ................................................................................................. 37 4.7 M icrowave Photoconductance Decay......................................................................... 39 4.8 Photolum inescence Im aging ........................................................................................ 39 5 Experim ental Results............................................................................................................ 41 5.1 Introduction...................................................................................................................... 41 5.2 Sample M ass and Thickness........................................................................................ 41 5.3 Phosphorus Diffusion Tim e-Temperature Profiles..................................................... 42 5.4 Measured Minority Carrier Lifetime and Interstitial Iron Concentration.................... 44 5.5 M icrowave Photoconductance Decay Results ............................................................. 46 5.6 Photolum inescence Imaging ........................................................................................ 51 6 Discussion ................................................................................................................................. 6.1 Introduction...................................................................................................................... 55 6.2 High-Temperature PDG Effectively Removes Interstitial Iron.................................... 55 6.3 Lifetim e Decreases with Higher PDG Temperature................................................... 57 6.4 Spatially Resolved Lifetim e - g-PCD ......................................................................... 59 6.5 Spatially Resolved Lifetim e - PL Imaging.................................................................. 63 6.6 Candidate Sources of High-Temperature Degradation................................................ 65 6.7 Furnace Cool Rate Verification ................................................................................... 66 6.8 Baseline Gettering at 845 'C ....................................................................................... 67 6.9 Conclusion ....................................................................................................................... 69 References.................................................................................................................................... 8 55 71 FIGURES Figure 1.1: Schematic of Photovoltaic Device ........................................................................ 14 Figure 1.2: Schematic of Photovoltaic Device Layers............................................................. 14 Figure 2.1: Simulated Efficiency vs. Metal Impurity Concentration....................................... 18 Figure 2.2: High-Temperature PV Processing Steps ............................................................... 20 Figure 2.3: Typical Phosphorus Diffusion Gettering Time-Temperature Profile..................... 20 Figure 2.4: Schematic of Iron during Phosphorus Diffusion Gettering.................................... 21 Figure 2.5: Solubility, Segregation and Diffusivity of Iron as a Function of Temperature.......... 22 Figure 3.1: Impurity-to-Efficiency Simulation Structure ....................................................... 24 Figure 3.2: Transient Response of Interstitial Iron by Plateau Time....................................... 29 Figure 3.3: Transient Response of Precipitated Iron by Plateau Time .................................... 29 Figure 3.4: Transient Response of Interstitial Iron by Plateau Temperature............................ 30 Figure 3.5: Transient Response of Precipitated Iron by Plateau Temperature ......................... 30 Figure 4.1: Silicon Sample Selection Schematic ...................................................................... 34 Figure 4.2: Typical Phosphorus Diffusion Profile.................................................................... 38 Figure 4.3: Loading the Tystar Furnace for Phosphorus Diffusion......................................... 38 Figure 5.1: Experimental Time-Temperature Profile for 950 *C Diffusion ............................. 42 Figure 5.2: Experimental Time-Temperature Profile for 1100 *C Diffusion.......................... 43 Figure 5.3: Samples Gettered at 900 *C and 1100 *C .............................................................. 44 Figure 5.4: i-PCD Maps for 92% Ingot Height ...................................................................... 47 Figure 5.5: p-PCD Maps for 95% Ingot Height ...................................................................... 48 Figure 5.6: Lifetime Histograms for 92% Ingot Height Samples............................................ 49 Figure 5.7: Lifetime Histograms for 95% Ingot Height Samples.............................................. 50 Figure 5.8: PL Image for 92% Ingot Height Samples .............................................................. 52 Figure 5.9: PL Image for 95% Ingot Height Samples .............................................................. 53 Figure 6.1: Reduction in Interstitial Iron Concentration.......................................................... 56 Figure 6.2: Percent Reduction in Interstitial Iron Concentration.............................................. 57 Figure 6.3: Percent Reduction in Interstitial Iron Concentration.............................................. 58 9 Figure 6.4: Post-Gettered Lifetime by Gettering Temperature................................................. 59 Figure 6.5: Sub-Region Selection for Lifetime Comparison 95%............................................ 61 Figure 6.6: Sub-region selection for Lifetime Comparison 92%.............................................. 61 Figure 6.7: Post-Gettered Lifetime Degradation by Temperature ........................................... 62 Figure 6.8: PL Image of Sister Samples by Gettering Temperature......................................... 64 Figure 6.9: Derivative of Mean Furnace Temperature during 950 *C PDG............................. 66 Figure 6.10: Derivative of Mean Furnace Temperature during 1100 *C PDG......................... 66 Figure 6.11: Average Lifetime Post-PDG by Temperature (92% Ingot Height)...................... 68 Figure 6.12: Average Lifetime Post-PDG by Temperature (95% Ingot Height)...................... 68 10 TABLES Table 3.1: Depth of Phosphorus Diffused Layer by PDG Temperature (25 min Plateau)..... 25 Table 3.2: Final Iron Concentrations by Plateau Time ............................................................ 27 Table 5.1: Sam ple M ass and Thickness.................................................................................. 41 Table 5.2: Mean As-Grown Lifetime and Interstitial Iron........................................................ 45 Table 5.3: Distribution of Samples by Ingot Height and Gettering Temperature ................... 45 Table 5.4: Mean Phosphorus-Diffused Minority Carrier Lifetime, r Fe-B, (ps)..................... 45 Table 5.5: Mean Phosphorus-Diffused Interstitial Iron Concentration [Fei] (cm- 3 ) ................. 45 Table 6.1: Slope of Lifetime Degradation by Temperature..................................................... 63 Table 6.2: Candidate High-Temperature Contaminants .......................................................... 65 11 12 CHAPTER 1 PHOTOVOLTAIC ENERGY 1.1 Introduction Global energy consumption is increasing [3], [4]. As a result, reducing the carbon intensity of energy produced is critical [5]. Energy is essential to quality of life [6], yet meeting growing energy demand is in increasingly challenging. Engineers will need to provide sustainable energy solutions and minimize the imprint of non-renewable energy. Photovoltaic energy offers a low carbon alternative to conventional energy sources [7], [8]. Solar energy is an immense energy resource. Each year over 40 Zeta Joules (1021 joules) of solar energy are incident on the U.S. [9]. Photovoltaic technology is one promising alternative to provide economic, sustainable energy for the future [10]. 1.2 Photovoltaic Basics A photovoltaic device converts light energy directly to electric energy. Light from the sun or other source is absorbed by the semiconductor device. The light energy in the form of photons excites free carriers enabling the carriers to move within in the semiconductor material. A basic schematic in Figure 1.1 shows light absorption by a typical solar cell device. 13 Figure 1.1: Schematic of Photovoltaic Device Light absorbed by the device is converted to eclectic current. Front and rear contacts interface with the semiconductor and direct the electric current through a load. Simple solar cell architecture consists of multiple layers designed to move generated current across a load. A cross-section of a typical simple solar cell device is illustrated in Figure 1.2. The semiconductor material consists of positively and negatively charged regions or the bulk and the emitter. The bulk is p-type silicon doped with boron (B-). The emitter is n-type silicon doped with phosphorus (P+). The two sections are joined forming a p-n junction and creating an electric field to move free carriers. Front and rear contacts interface with the semiconductor material and carry charge from the cell to an external load. Front Contact Emitter Bulk Back Contact Figure 1.2: Schematic of Photovoltaic Device Layers Simple PV device architecture consists of semiconductor material doped to form a p-n junction. The bulk is p-type doped with boron (B-). The emitter is n-type doped with phosphorus (P+). 14 When designing and manufacturing photovoltaic devices, the goal is for as many electrons generated by absorbed photons to flow towards and reach the terminals of the solar cell [11]. High quality semiconductor materials are essential for efficient devices as explained in Chapter 2. Manufacturing processes must be optimized to maintain material quality and ultimate device efficiency at a competitive cost. This thesis explores one step of the manufacturing process, phosphorus diffusion gettering, typically used for the dual purpose of improving material quality and doping the n-type emitter layer. Specifically, performing phosphorus diffusion at high-temperature which is promising for removal of interstitial iron impurities was simulated (Chapter 3) and tested experimentally (Chapter 4-6). 15 16 CHAPTER 2 IRON IN SILICON 2.1 Silicon PV Material Silicon is the majority semiconductor material for PV production today. In 2008, silicon comprised of 83% of manufactured PV (35% is mono-crystalline and 48% is multicrystalline silicon material) [12]; in 2013, preliminary numbers suggest silicon comprised 91% of manufactured PV. While silicon technology dominates, the energy market drives innovation toward increasing cell efficiency and reducing cost to reach grid parity. Manufacturing costs must be controlled to reach the SunShot goal of $0.06/kWh by 2020 [13]. To achieve this goal, a key strategy is to increase efficiency, as efficiency is the principal parameter influencing ultimate energy cost [14]. 2.2 Silicon Material Purity From the integrated circuit industry to photovoltaic applications, maintaining material quality in silicon is critical [15]. Even low concentrations of metal impurities can degrade the performance of semiconductors [16]. Impurity concentrations as low as 1 x 1012 atoms/cm 3 diminish device performance [17]. Electronic grade silicon is maintained at nine nines purity (99.9999999%) and silicon grade silicon is maintained at six nines purity (99.99999%). For photovoltaic devices, dissolved or precipitated metal impurities hinder device performance by creating recombination centers [17]. Experimentally, the Westinghouse study showed that metals reduce device efficiency by reducing diffusion length [18]. Metals including Fe, Cr, Co, Ni, and Cu have been shown in simulation to reduce cell efficiency after exceeding a certain concentration illustrated in Figure 2.1 [19]. Iron is particularly detrimental to p-type silicon photovoltaic device performance [20], [21]. 17 21 CU 20 19 Cr 118 'WE17 16 15 14 (a) p-Sl bae 100 " a'A 1011 1012 1013 1014 101 i 101 Metal impurity concentration [cm-] Figure 2.1: Simulated Efficiency vs. Metal Impurity Concentration Simulated PERC device shows decrease in efficiency with increasing metal impurity concentration. Each metal impurity impacts efficiency at a different concentration. (Reprinted from [19] with permission 2011 IEEE) Beyond the concentration of metal in the silicon, the distribution of metal within the sample is critical to consider [22], [23]. Iron is present in multiple forms including point defects and precipitates [23]. Iron precipitates reduce minority carrier diffusion length due to large capture cross section [24]. Iron point defects are more detrimental than precipitates by concentration because for the same concentration, point defects are distributed throughout the bulk [23]. In addition, increased iron concentration has been shown to increase the crystal defect concentration in the ends of contaminated ingots [25]. Iron is also industrially relevant, as concentration of iron in typical commercial solar materials is sufficient to degrade performance significantly [26]. 2.3 Silicon Manufacturing Process Impurities are introduced to the material throughout the manufacturing process. Green summarizes the manufacturing process in 5 steps: 1. Reduction of silicon-bearing feedstock to metallurgical-grade silicon. 18 2. Purification of metallurgical-grade silicon to semiconductor grade silicon. 3. Conversion of semiconductor grade silicon to single crystal silicon wafers. 4. Processing of single-crystal silicon wafers. 5. Solar cell encapsulation into weatherproof solar cell modules [27], [28]. The manufacturing process for multicrystalline silicon is analogous. For multicrystalline samples, introduction of impurities begins in the feedstock material, crucible and liner [29]. During subsequent high-temperature steps, the total and local concentrations of iron are manipulated. 2.4 Iron States in Silicon Iron is found in silicon in predominantly two forms: as interstitial iron, Fei, point defects distributed throughout the lattice or as metallic precipitates, Fep, an agglomerated form. Total iron concentration in most industry-relevant samples is approximately the sum of both the interstitial and precipitated iron concentration. Typically, the majority of iron is present in precipitated form [30]. Iron in both precipitated and interstitial states impacts material lifetime. Interstitial iron is usually the lifetime limiting defect as the recombination activity of precipitated iron is much lower [22]. In reality, the effect of iron on lifetime is dependent on both interstitial and iron precipitated concentration [31]. Furthermore, the distribution of iron within multicrystalline silicon must be considered. 2.5 Gettering Gettering is a thermal and chemical processing step used to remove metal impurities from a semiconductor. Gettering techniques are used throughout the semiconductor industry to move metal impurities away from the active device region. In the integrated circuit industry, gettering is used to remove defects from the active surface. In the photovoltaic industry, gettering is used to remove metals from the bulk of the material to the surface. Phosphorus diffusion gettering (PDG) is a well-established step in the solar industry for removing impurities [32]. Gettering is one in a series of high-temperature steps performed when manufacturing a solar module. Thermal process steps summarized in Figure 2.2 are: crystallization growth, phosphorus diffusion gettering, anti-reflection coating (ARC), and 19 metallization firing. While gettering is optimized to dope the sample and increase material quality, subsequent thermal steps must also be considered to maintain final material quality. Cell Processing Schematic: High-temperature Steps 1414 850 a) E 400 Growth (20 hrs) PDG (1.5 hrs) ARC (10 min) Metallization (10 min) Figure 2.2: High-Temperature PV Processing Steps Summary of temperature and duration of each high-temperature step performed during manufacturing of a PV module. (Adapted from [33]). The typical phosphorus diffusion gettering process is broken down further into a series of three steps illustrated in Figure 2.3. First, the furnace temperature is increased from loading temperature. Second, the phosphorus diffusion step is performed isothermally. Third, the furnace temperature is lowered at a controlled rate. P-Diffusion 2 o E 2 1 3 Time Figure 2.3: Typical Phosphorus Diffusion Gettering Time-Temperature Profile Typical profile is constituted of three steps: temperature ramp, isothermal phosphorus diffusion step, and cool down. (Reproduced from [34]). 20 The phosphorus diffusion step serves a dual purpose during manufacturing of typical p-type semiconductor material. The phosphorus in-diffusion into the silicon dopes the outermost layer of the silicon n-type. In addition, the phosphorus diffusion step getters metal defects to the phosphorus layer improving the bulk material quality. Within the bulk, the phosphorus diffusion step has a direct impact on the distribution of iron and phosphorus concentration. As the temperature and phosphorus concentration change the solubility and diffusivity of iron in silicon are impacted. The effect of gettering on iron particles is illustrated by the schematic in Figure 2.4. 1. Fe precipitates dissolve 2. P diffuses in, Fe moves to P layer 3. P diffuses further, Fe moves to P layer Figure 2.4: Schematic of Iron during Phosphorus Diffusion Gettering Progression of iron concentration during the three steps of phosphorus diffusion gettering. (reproduced from [34]) First, as the temperature of the furnace increases, the solid solubility of iron in silicon increases. As a result, iron precipitates begin to dissolve and interstitial iron concentration increases. Second, phosphorus is in-diffused into the sample forming an emitter layer (typically at constant temperature). The solid-solubility of iron in the phosphorus layer is higher than in the bulk of the material. Gettering occurs as interstitial iron diffuses from the bulk of the material into the phosphorus-diffused region. Further, as iron is drawn out of the bulk, iron precipitates continue to dissolve to meet the solid solubility concentration for interstitial iron. Third, the temperature of the furnace is lowered. As the temperature decreases, the solid solubility of iron in the bulk decreases. The solid solubility of iron in the phosphorus diffused emitter also decreases, but at a slightly lower rate resulting in a strong gettering iron. After a given point, the solid solubility and diffusivity decrease to a point below which gettering is no longer effective. 21 7-1 X10 6 1x101 , F E x 4 1.6 51.2 0_ I.-. 0 0.8 _0 -~20 700 750 800 850 ?0 Temp. (*C) 750 800 Temp. (*C) 850 700 750 800 850 Temp. (*C) Figure 2.5: Solubility, Segregation and Diffusivity of Iron as a Function of Temperature Solubility and diffusivity of ion in silicon increase as a function of temperature. Simultaneously, the segregation coefficient decreases a function of temperature. (Reproduced from [34]). 2.6 Designing a Gettering Profile A gettering profile must be designed taking into account the non-linear behavior of iron in silicon as a function of temperature. The complex parameter space of iron in silicon is further influenced by initial material quality and constrained by processing time, manufacturing constraints and furnace limits. Many strategies have been employed to attempt to find the ideal gettering profile. Multiple authors have optimized the gettering profile to reduce processing time [34], [35], [36]. Others have considered the initial material properties [31], [37]. While yet others have attempted to concentrate iron in precipitates during thermal steps a process called precipitate ripening [38], [39]. Most recently, computation methods including a genetic algorithm have been used to probe the complex iron-silicon parameter space [40]. Mathematical model, simulation tool and gettering optimization are discussed further in Chapter 3. 22 CHAPTER 3 SIMULATED IRON RESPONSE TO GETTERING TIME-TEMPERATURE PROFILE 3.1 Motivation for Simulation Simulations provide invaluable insight into the complex problem of reducing defect concentration in silicon. The distribution of iron in silicon changes as a function of the thermal profile the material is subjected to. While experiments are useful for characterizing and understanding material quality, simulations provide other advantages and unique information. Simulations are much faster and cheaper than the equivalent experiment, allowing researchers to test ideas before devoting hours and materials. More effective experiments are designed after using simulations to iterating through parameters space. From theory and interpolation, simulations can even provide information and insight that is not available experimentally. 3.2 Model of Iron in Silicon The Impurities-to-Efficiency Simulator (12E) developed by Hofstetter et al. [2] is a model used to predict the impact of initial contaminate concentration and processing conditions on the final material lifetime and cell efficiency. 12E currently runs in MATLAB. Designed for optimizing the phosphorus diffusion gettering process, the simulator takes material, process and cell structure inputs and outputs iron profiles, lifetime, and efficiency. 23 Impurity-to-Efficiency Simulator Fe content & distribution N F Profe segrigusion, R,/R, F-dffn, Proc.It Figure 3.1: Impurity-to-Efficiency Simulation Structure Summary of I2E inputs, outputs, and model. (Reprinted from [41]). The program is structured in three steps. First, partial differential equations (PDE) governing the behavior of iron are solved for a given set of inputs conditions and time-temperature profile to determine the post-gettered iron distribution. Second, the program calculates minority carrier lifetime for the iron distribution using Shockley-Reed-Hall statistics. Third, the program uses PCID [42] to calculated device efficiency for the iron distribution. In I2E, the partial differential equations governing the behavior of iron in silicon are solved using a ID finite element method. The domain for the simulation is taken as the thickness of the wafer and divided using a logarithmically spaced meshed in x. The logarithmic spacing provides higher resolution near the surface of the wafer so that phosphorus diffusion and resulting segregation can be more accurately captured. Mesh spacing can be adjusted. For all simulations, time step size is scaled with mesh spacing. At each step, the distribution of iron in the system is described by two variables: interstitial iron concentration, C,, and iron precipitate radius, r. The diffusion of phosphorus into the wafer is described by the concentration of phosphorus, P. All three variables are calculated for each time step at every mesh point. The concentration of phosphorus in silicon is calculated using Fick's Law, (3.1, developed by Bentzen et al. [43]. D is the diffusivity of phosphorus is silicon and an exponential function of temperature [2]. 24 a[P] at _ a ax ( a[P] D([P]) (3.1) The law describes the diffusion of phosphorus atoms due to local phosphorus concentration and temperature. Phosphorus diffusivity depends exponentially on temperature. With increasing temperature, the depth of the phosphorus doped emitter layer increases as reported for a 25 minute simulated PDG plateau time in Table 3.1. The increased depth of the emitter layer defined as the p-n junction is important for the gettering, processing, and device performance (blue response, sheet resistance, and contact resistance). If the emitter needs to be removed after gettering (such as for lifetime measurements), the depth of the emitter is notably deeper. Table 3.1: Depth of Phosphorus Diffused Layer by PDG Temperature (25 min Plateau) Temperature (*C) Phosphorus Depth (pm) 850 900 950 1000 1050 1100 0.5 0.75 1.0 1.5 2.3 3.5 The dynamics of iron are modeled for both the interstitial and precipitate states. Total iron concentration is the sum of the concentration of interstitial iron and concentration of precipitated iron. Each form of iron can be modeled as a function of temperature and distribution. Interstitial iron diffuses though the silicon lattice. The diffusion of interstitial iron in silicon is modeled using kinetics and model described by Tan et al. [44]. The diffusion coefficient of iron in silicon, DFe, is a function of temperature. The segregation coefficient is a. ac_ -xa (ac - DFa C1aau (3.2) 25 Segregation of iron in the phosphorus layer is described by the model developed by Haarahiltunen et al. [45] where Vsi are vacancies, K are constants, and n is the electron concentration. cxx)=n(x) O() = 1 + VsiKeq,i ni (1 + Keq, 2 [P(X)]) (3.3) Iron precipitates are modeled as spheres. With changing iron solubility, the precipitate will dissolve or the precipitate radius, r, will decrease. The change in precipitate radius is described [2], [46] with equation (3.4). Or (Ci -t = VFeDFe Ot - Ceq,Fe) (34) (34 r With increasing temperature, solid-solubility of iron in silicon increases and precipitated iron dissolves increasing the concentration of interstitial iron. The increase in interstitial iron concentration is accounted for with equation (3.5) [2]. aC, = 41rNrDFe(Ceq,Fe - C) (3.5) 3.3 Minority CarrierLifetime Minority carrier lifetime is the metric used to predict device performance. Lifetime can be expressed as a function of iron concentration for both interstitial and precipitated iron. For interstitial iron, lifetime is limited by free carriers recombining at interstitial point defects as described by Shockley-Read-Hall Statistics [47], [48]. Minority carrier lifetime is inversely proportional to interstitial iron concentration (a is the minority carrier cross section and Vth is the thermal velocity of minority carriers) [2]. 1 = T- CiOth 26 (3.6) For iron precipitates, lifetime can be expressed as a function of precipitate radius as described by equation (3.7) [46]. 1 TP = 41rr2 N sD/r s + D/r (3 7) In the impurities-to-efficiency simulator, lifetime and efficiency are calculated. Lifetime measurements can also be averaged using multiple different means as described by Wagner et al. [49]. Lifetime measurements are added harmonically. 1 1 1 (3.8) 3.4 Simulation of High-Temperature Gettering To determine the effect of high-temperature gettering on iron distribution in silicon, hightemperature gettering processes were simulated. To understand the marginal effect of increasing temperature on iron distribution, simulations were run at incrementally higher temperatures. Since in experimental setup higher temperature gettering profiles typically require a longer total time (for ramp up and down), gettering with incrementally longer plateau lengths was also simulated. For both sets of simulations, all other parameters were held constant between the simulations including: load temperature, unload temperature, temperature ramp-rate, temperature cool-rate. Initial iron concentration was held constant at with a total iron concentration of 1 x 1015 cm-3 and initial precipitate radius of 25 x 10-7 cm. From the simulations, the final concentration and distribution of are compared in Table 3.2. Table 3.2: Final Iron Concentrations by Plateau Time Plateau time 7 4.1 101 5.8 Interstitial Iron Concentration 4.3 x 10 Precipitated Iron Concentration 6.5 x 60min 40min 25min 7 x 10 3.6 x 107 101 5.0 x x 101 27 3.5 Transient Response To better understand the impact of gettering parameters on iron during gettering, the transient response of iron to the gettering time-temperature profile was plotted. While final values are useful for determining ultimate impact on a solar cell, the transient response offers insight for gettering plateau optimization. The effect of each section of the time temperature profile is clearly illustrated by transient response plots. To create transient plots, iron concentration in the bulk for each time step was calculated and saved. (Calculation is preformed using Function transFE.m, written in Matlab and included in the appendix). Transient response of interstitial and precipitated iron to gettering profiles of varying plateau duration are compared in Figure 3.2 and Figure 3.3. Immediately apparent, longer plateau times result in reduced precipitated radius, as the precipitates are held at high-temperature longer with more time to dissolve and getter. To test temperature dependence of gettering, profiles were shifted upward. Ramp and cool rates and plateau time are held constant. Transient iron response to increasing plateau temperature is compared in Figure 3.4 and Figure 3.5. As the gettering temperature increases, the precipitate rate of dissolution is much faster. Higher gettering temperature increases solid solubility of iron resulting in increased precipitate dissolution and iron gettering. With increased plateau temperature, the final concentration of both interstitial and precipitated iron is lower. The solid solubility of iron in silicon increases with increasing temperature, allowing more precipitated iron to dissolve into the bulk. While the peak interstitial iron concentration is higher, the final interstitial iron concentration is lower provided sufficient cooldown time to getter iron from the bulk. 28 Transient Response x 101, of Interstitial Iron -25 - -40 -60 1 2- 1 0- 8- it 6-4 2- 0 20 40 60 80 Time [min] 100 120 140 160 Figure 3.2: Transient Response of Interstitial Iron by Plateau Time As the plateau time increases, the interstitial iron has a longer time to getter for a lower final concentration. Transient Response of Iron Precipitates x 1" V -- 8.5k -25 40 60 8 7.5 7 6.5 6 5.5- 0 20 40 60 80 100 120 140 160 Time [min] Figure 3.3: Transient Response of Precipitated Iron by Plateau Time As the plateau time increases, the final precipitated iron concentration decreases. More precipitated iron is gettered during longer plateaus. 29 Transient Response of Interstitial Iron x 1011 4 F-- -800 .-- - 850 -900 3.5 3 2.5 2 - U- 1.5 1 0 20 40 60 80 100 120 140 Time [min] Figure 3.4: Transient Response of Interstitial Iron by Plateau Temperature With increased plateau temperature, more interstitial iron is dissolved into the bulk and gettered. Transient Response of Iron Precipitates X 102 800 850 9001 8.5 8 7.5 7 6.5 6 5.5' 0 20 40 80 60 100 120 140 Time [min] Figure 3.5: Transient Response of Precipitated Iron by Plateau Temperature With increased gettering temperature, solid solubility of iron increases. Precipitates dissolve faster at higher temperature. 30 3.6 Other Impurities in Silicon Other metals have been shown to degrade the material quality of silicon[19]. Impurities can be modeled similarly by changing the model parameters for diffusivity and solubility as a function of temperature. 31 32 CHAPTER EXPERIMENTAL METHODS FOR HIGH-TEMPERATURE PHOSPHORUS DIFFUSION GETTERING 4.1 Introduction High-temperature phosphorus diffusion gettering (PDG) experiments were performed to determine the effect of high-temperature PDG on iron concentration and minority carrier lifetime. Three experiments were performed at varying gettering temperature on multicrystalline silicon samples of two iron concentrations. 4.2 Multicrystalline Silicon Samples For the high-temperature PDG experiments, multicrystalline silicon samples were chosen as illustrated in Figure 4.1. The samples are from two sets of three sister wafers, i.e. from vertically adjacent ingot heights. Three sister wafers from two different ingot heights (92% and 95%) were used to maintain similar grain structure and iron concentrations within the sample sets. From ICPMS data fitted with the Scheil equation, the 92% ingot height wafers have an estimated total iron concentration of 7.4x1013 cm3, and those at 95% ingot height have a concentration of 1.4x10" cm. 33 1 1 Ingot Saw sister wafers cut 3 3 5 5 Brother wafers Figure 4.1: Silicon Sample Selection Schematic Sister wafers, or adjacent wafers, are selected from are cut from two ingot heights. Brother samples, from the same wafer, are cut from each sister wafers. Each sister wafer was cut into smaller pieces or samples using a laser cutter and laser scribed with wafer and sample number. Three brother samples were chosen from each of six sister wafers for a total of 18 samples. 4.3 Sample Preparation - Saw Damage Etch Before performing PDG experiments on the samples, as-grown minority carrier lifetime and interstitial iron concentration were measured. The as-grown measurements serve as a baseline for comparison with the post-gettered measurements. Samples were first cleaned and passivated in preparation for minority carrier lifetime measurement (procedures in Appendix 1). Multicrystalline wafers are sliced from an ingot using a sawing process that damages the surface of the wafers. The damaged surface is contaminated with high concentrations of impurities from the sawing slurry. The impurities must be removed to avoid contaminating the bulk of the material during subsequent high-temperature processing steps. The 10 gm damaged layer of silicon is removed using a CP4 etch chemical process outlined (full procedure in Appendix 1): 34 1. Dip in Hydrofluoric Acid (HF) for one minute to remove native oxide. 2. CP4 chemical bath to remove saw damage. Bath consists of a 15:5:2 ratio of 70% nitric acid, 100% acetic acid, and 49% HF. 3. Dip in HF to remove oxide formed during CP4. 4. Alkaline quench to remove porous silicon. Quench consists of 30% KOH by weight. 5. Dip in HF to remove oxide formed during KOH dip. The depth of the CP4 etch is controlled by the time duration that the samples are submersed in the bath. Longer bath durations remove more material. The time to depth removed is nonlinear as the reaction is exothermic. The etch rate increases as the temperature of the bath increases. The temperature of a bath will increase faster with more samples than a bath with fewer samples. To verify that the bath time was sufficient to remove all the saw-damaged silicon, the mass, m, of a sample is measured before and after CP4. The final thickness is estimated by assuming that the sample surface area is known and remains constant. The sample cross sectional area, A, is estimated from the dimensions of the sample. The density of silicon, p, is estimated as 2.329 g/cm 3. Thickness for a given sample mass is calculated using equation (4.1). (4.1) - t= m pA 4.4 Minority CarrierLifetime Measurement Minority carrier lifetime is measured to determine the quality of the material before and after phosphorus diffusion gettering. From the minority carrier lifetime measurements, the interstitial iron concentration can be calculated (section 4.5). In preparation for the measurement, the samples are cleaned. In addition, the sample surface is passivated with aluminum oxide to reduce recombination at the wafer surface [50]. RCA Surface Clean and ALD Deposition procedures included in the Appendix. Sample minority-carrier lifetime is measured using the Sinton WCT-120 Photoconductance Lifetime Tester using the quasi-steady-state photoconductance technique (QSSPC). The tool illuminates the sample with a flash bulb, resulting in photogeneration or an injection of minority 35 carriers. The tool measures the conductance and corresponding incident light intensity. The lifetime is calculated from the slope of the photconductance decay curve. [51]. The effective lifetime, Teff, is a function of excess carrier density, An, and the carrier generation rate, G, and the time derivative of excess carrier density. The effective lifetime can be expressed as [52]: dAn Teff(An) = G(t) (4.2) -dt For the multicrystalline samples considered, the lifetime is much lower than the 2.3 ms typical flash decay time constant [52]. The effective lifetime simplifies using the quasi-steadystate assumption to: Tefff(An) = An -(4) G(t) (4.3) Excess carrier density is calculated as a function of time from measured sample conductivity. Conductivity is measured using an RF coil. Generation rate is calculated as a function of time from the measured light intensity. Light intensity is measured using a built in light sensor. Additional specifications for the Sinton WCT-120 tool are found in the manual [53]. 4.5 Interstitial Iron Quantification by FeB Dissociation Interstitial iron concentration in p-type silicon is measured from change in minority carrier lifetime for dark and illuminated samples. The method proposed by Zoth and Bergholtz for detecting low concentration of metal impurities contaminating silicon in the integrated circuit industry is capable of detecting interstitial iron concentrations as low as 2-5 x 1011 cm [54]. The measurement method has many advantages over measurement methods such as DLTS or SIMS including the sensitivity, contactless, non-destructive and rapid. The measurement takes advantage of the equilibrium reaction for iron and boron in silicon: Fet + B- ++FeB 36 (4.4) Interstitial iron in silicon is mobile at room temperature. As the iron is positively charged it forms a covalent pair with the negatively charged boron. measurement and dissociated FeB pairs are associated for dark illuminated measurements during pair in p-type silicon. Manipulation of the Shockley-Read-Hall equation allows calculation of iron concentration from measured associated and dissociated lifetime [34], [54], [55]. (4.5) [Fei] = C ( diss assoc) 4.6 Phosphorus Diffusion Phosphorus diffusion is performed in a Tystar Mini-Tytan 3800 diffusion furnace. The samples are placed into a quartz furnace tube and loaded into the pre-heated furnace. The furnace is pre-programmed with time-temperature and gas flow profiles. Typically the program is as follows: e Pre-heat - The furnace is heated from standing temperature at 500 *C to load temperature at 800 'C. e Load - The furnace tube opens allowing samples to be loaded. After loading samples, the tube is closed and the program allows the furnace temperature to stabilize. e Ramp-up - The temperature of the samples is increased from the load temperature at 800 *C to the diffusion temperature at 4 *C/min. * Phosphorus diffusion - POC13 gas is flowed into the furnace tube constant temperature. To fill or flush the furnace tube with gas requires 6 minutes (or 12 minutes for a complete changeover). Typically, the gas is turned over twice for a total time of 24 minutes. " Ramp-down - The temperature of the furnace is decreased from the diffusion temperature to unload temperature at 550 *C at 4 *C/min. e Unload - The furnace tube opens allowing samples to be unloaded. The typical time-temperature profile and gas flow schedule is illustrated in Figure 4.2. 37 900 850 a GD 800 750 700 650 600 500 0 10 20 --30 40 50 60 70 80 Time (min) Gas N2 POC 3 N2 Figure 4.2: Typical Phosphorus Diffusion Profile Typical phosphorus diffusion time-temperature profile and corresponding gas flow. Figure 4.3: Loading the Tystar Furnace for Phosphorus Diffusion Samples are centered on the rails when loaded into the furnace at 800 *C. Large insulating plug (lower right) reduces heat loss to maintain the furnace temperature. 38 4.7 Microwave Photoconductance Decay Microwave photoconductance decay (p-PCD) measures spatially resolved minority carrier lifetime. The Semilab WT-2000 tool uses the same theory as described in section 4.4 for the minority carrier lifetime measurement. To excite carriers, an infrared semiconductor laser scans the surface of the sample generating free carriers. Simultaneously, the tool measures microwave reflectivity which is proportional to the sample conductivity [56]. As the laser scans over the surface of the sample, the tool creates a raster or map of the effective lifetime at each pixel. The spatially resolved lifetime map is particularly useful for comparing the effect of gettering on particular grains or regions of the sample. 4.8 Photoluminescence Imaging Photoluminescence imaging (PL) produces a high-resolution map of carrier lifetime rapidly and non-destructively [57], [58]. The map illustrates the spatial distribution of lifetime in the sample. A PL image is obtained by illuminating a sample with a 808 nm laser to excite minority carriers. Simultaneously a Princeton Instruments/ACTON, PIXIS: 1024 series infrared camera is used to capture the luminescence of the sample. 39 40 CHAPTER 5 EXPERIMENTAL RESULTS 5.1 Introduction To determine the effect of high-temperature phosphorus diffusion gettering on multicrystalline silicon, diffusion processes were performed at three temperatures. Sample minority carrier lifetime and iron concentration were measured before and after the diffusion process as a metric for material quality. Spatially resolved measurements provide further insight into the uniformity of the impact on each sample. 5.2 Sample Mass and Thickness To verify the thickness of sample material removed during CP4 etch, sample masses were recorded. From the mass the thickness was calculated. Example recorded mass and calculated thickness values are reported in Table 5.1. Table 5.1: Sample Mass and Thickness Mass [g] Procedure Pre-CP4 950 *C (as-grown) 1100 *C (as-grown) -- 0.7790 Post-CP4 Thickness [microns] Pre-CP4 0.6282 0.6890 -- 176.0 Post-CP4 146.6 155.7 41 5.3 Phosphorus Diffusion Time-Temperature Profiles To determine the effectiveness of high-temperature phosphorus diffusion gettering, three high-temperature profiles were designed for experimental tests. Diffusion temperature is the experimental variable. To isolate the impact of temperature, experimental profiles had consistent load temperature, ramp rate, diffusion time, cool rate and unload temperature. Profile design and theory are described in Chapter 3. Experimentally, time-temperature profiles are pre-programmed into and controlled by the furnace computer. The temperature is measured by three thermocouples along the length of the furnace tube (at the front, middle and rear) to confirm a uniform tube temperature. Recorded time-temperature profiles from the center thermocouple for each of the two higher temperature experiments are reported in Figure 5.1 and Figure 5.2. 1000 goo 850 S800 750 700 650 600 550 500 0 20 40 60 80 100 120 140 160 180 Time (min) Figure 5.1: Experimental Time-Temperature Profile for 950 *C Diffusion Temperature recorded by furnace center thermocouple. 42 1200 1100 S1000 E 800 700 600 - 500 0 50 100 150 Time (min) 200 250 Figure 5.2: Experimental Time-Temperature Profile for 1100 *C Diffusion Temperature recorded by furnace center thermocouple. Experimental profiles deviate slightly from the ideal simulated profiles. During loading the temperature of the furnace drops below the specified temperature because the tube is open to the environment. idealized When ramping the furnace temperature, the ramp rate is often slower than the 1 00C/minute for several reasons. The furnace temperature controller prioritizes maintaining a uniform furnace temperature over the ramp rate. If the measured temperature of a zone of the furnace deviates too far from the other zones, the controller slows the ramp rate to allow the tube to re-equilibrate. At the transition to the constant temperature plateau, the furnace ramp rate slows as the furnace controller attempts to stabilize the constant temperature in all three zones. The controller does not proceed to the phosphorus diffusion step until all three zone temperatures are uniform to within 1PC for one minute. 43 Figure 5.3: Samples Gettered at 900 *C and 1100 *C Samples from adjacent wafers exhibit similar grain structure after gettering at 900 'C (left) and 1100 *C (right). The sample gettered at higher temperature appears blue due to thicker emitter layer formed during higher temperature phosphorus diffusion. 5.4 Measured Minority Carrier Lifetime and Interstitial Iron Concentration To measure and quantify the effectiveness of high-temperature phosphorus diffusion gettering profiles, the minority carrier lifetime was measured and interstitial iron concentration was calculated for each sample before and after gettering. As-grown mean lifetime, interstitial iron and total iron concentration are reported in Table 5.2. Mean as-grown measurements are reported by ingot height. Also total iron concentration was measured by inductively coupled plasma mass spectrometry (ICPMS) fitted with the Scheil equation. Measured lifetime and iron concentrations are consistent within each of the sample sets as established by low standard deviations. 44 Table 5.2: Mean As-Grown Lifetime and Interstitial Iron [Fei] (cm 3 ) 1.13 7.4 10" Mean 92 39.9 6.1 2.67 Mean 95 5.78 0.96 2.83 + 0.42 x 1012 x [Fettai] (cm~ 3 ) T FeB (js) x 10" 1.4 x 1014 After characterizing the as-grown iron concentration and lifetime values of the sample set, the samples were split into three groups for high-temperature phosphorus diffusion gettering at three plateau temperatures. Sets of sister wafers from both ingot height were randomly assigned to the three gettering temperatures summarized in Table 5.3. Table 5.3: Distribution of Samples by Ingot Height and Gettering Temperature 900*C 950*C 1100*C 92% ingot height MG1090 MG1089 MG1088 95% ingot height MG1119 MG1118 MG1120 After gettering, lifetime and iron concentration of each sample was measured again. Average post-gettered lifetime for each ingot height is reported by PDG plateau temperature in Table 5.4. Table 5.4: Mean Phosphorus-Diffused Minority CarrierLifetime, T Fe-B, (ps) 1100 *C 950 C r (Rs) 900 *C Mean 92 85.5 + 29.4 72.3 28.9 26.8 Mean 95 78.5 74.9 16.8 18.5 + 3.5 26.7 0.3 Interstitial iron concentration is calculated from lifetime measurements and reported by PDG plateau temperature Table 5.5. ) Table 5.5: Mean Phosphorus-Diffused Interstitial Iron Concentration [Fei] (cm~ 3 [Fei] (cm 3 ) 900 *C 950 *C 1100 *C Mean 92 9.18 5.61 x 10' 9.90 11.4 x 109 9.47 8.82 x 109 Mean 95 1.27 0.97 x 1010 3.91 +3.00 x 109 2.03 1.90 x 1010 45 5.5 Microwave Photoconductance Decay Results Spatially resolved maps of lifetime were produced to provide a better understanding of the lifetime distribution. Each sample was mapped at high resolution (0.5 mm raster) using a 200 ns pulse width. After mapping all samples, each map was scaled to the same lifetime range of 9 to 104 As. Lifetime maps are shown for 92% and 95% ingot height samples in Figure 5.4 and Figure 5.5 respectively. From the p-PCD measurement, histograms illustrating the area-weighted distribution of lifetime for each sample were plotted. Histograms for the 92% and 95% ingot height samples are plotted in Figure 5.6 and Figure 5.7 respectively. 46 0 ACB 0 C) P CL 9 us 104 us Figure 5.4: p-PCD Maps for 92% Ingot Height Lifetime ranges from 9 to 104 gs. 47 A B C 0 0 003L 0 0 0 LO) 0) 0 0 0L 0 0 C!, 9 us 104 us 0 Figure 5.5: p-PCD Maps for 95% Ingot Height Lifetime ranges from 9 to 104 ps. 48 A B .......... ------------------------- c ----------- ----------------------- ......................... ....... .......... ------------- ................................... .. ------------------------- 0 0 ------- ------------ ---------- ----------- - 0) 0 ....... ..... 9 a 41 1. 47 a 1 ",V- w ) 9 20 es 47 L ............ C IN 1.) -- -------- 10 104 [W) ...... ............I ------------ ------------ ----------------------- LO 0 a ........... ------------------------ .......................... 20 41 M IN ----------- ------ M ----------- . ........ 15 w M ------------------------ 1. 20 ................... 20 9 IN 41 ----------- 7 ----------------------- ------------------------ - ------...... ...................... C> CD ...... ...... - ...... 15 ........... ........................ in .......I ......... I........... ........... 10 ------------ ........... ...... ........ .................... ... 0 0 a 0 47 W ... ... as 1" 0 9 M 47 w M 104 47 Be e5 IN LA4-1.1 M 9 us 104 us 0 Figure 5.6: Lifetime Histograms for 92% Ingot Height Samples Histograms of sample area at by lifetime. Lifetime ranges from 9 to 104 gs. 49 A B r ........... F........... 7 ........... T ........... ........................ c T........... --------- ............. ......... ................ ............ ,...... .......... ....... ........... 0 ........ 0 ) 0 20 47 M as '00 0 a 2S 104 a uft Low. 1-11 U'." "I ........... ....................... 7 ------------------------ 'a ------------ 7 ............ -T ......................... 10 as 10. 1.1 ........... 7 ....................... ......................... LO .. .......... ........ ........... 0 0 21 47 W 46 104 20 9 41 40 is" ... ... 104 9 26 ...... 30 ...... LO*w W ........... 7 .............. ........ 7 .... .. 1. 47 1w) ............. 3D ........... ........................ ......................... ........ .......... . ...... -- ----------- 1 ----------- 1----------- - ----------- ------- 0 26 47 05 Uf". 1.) 05 104 IQ ------- 9 29 47 ---------- a Lft ft IN 1........... ............ IQ 0 ----------- 1----- ----- --------- --- 9 1-1 M 9 us 28 47 66 Lb. 1.) 104 us 0 Figure 5.7: Lifetime Histograms for 95% Ingot Height Samples Histograms of sample area at by lifetime. Lifetime ranges from 9 to 104 gs. 50 es 104 5.6 Photoluminescence Imaging Photoluminescence imaging (PLI) produced a high-resolution lifetime image of each sample. PL images for the samples from 92% and 95% ingot heights are plotted in Figure 5.8 and Figure 5.9 respectively. Each sample was imaged at 60% laser power. The laser excites excess carriers in the sample, and a camera captures the emitted energy at recombination. Higher lifetime regions are brighter. In addition, PL images of higher-lifetime samples appear blurrier as minority carriers can travel farther before radiatively recombining. 51 CD NA U 0 0 0 0 Figure 5.8: PL Image for 92% Ingot Height Samples Brighter regions indicate higher lifetime; arbitrary scale. 52 U 0 0 0 0 Figure 5.9: PL Image for 95% Ingot Height Samples Brighter regions indicate higher lifetime; arbitrary scale. 53 54 CHAPTER 6 DISCUSSION 6.1 Introduction From experimental results, the impact of high-temperature PDG on multicrystalline silicon performance is determined. Previous work on high-temperature PDG has shown that increasing gettering temperature is effective at removing impurities and improving material quality to a point at which the material begins to degrade with increasing temperature [59]. Multiple possible defects and sources of degradation at high-temperature are considered including Fe, Cr, 0, and dislocation propagation. While some have considered iron a lifetime limiting defect for high-temperature annealing [59], experimental and simulated results show that at high-temperatures iron is successfully removed and not responsible for high-temperature degradation. In determining a true upper bound on high-temperature PDG, it is essential to investigate multiple possible sources of degradation. 6.2 High-Temperature PDG Effectively Removes Interstitial Iron High-temperature gettering is effective at reducing interstitial iron concentration. Experiments confirmed modeled predictions in Chapter 3 that high gettering temperature would reduce interstitial iron concentration. For each gettering temperature, the interstitial iron concentration was averaged by ingot height and graphed in Figure 6.1. For both ingot heights and all three gettering temperatures, 55 iron concentration was reduced to below 1 x 1010. The measured iron concentration is approaching the lower bound for the tool and QSSPC technique so the actual iron concentration could be lower. Most important is that the effective iron concentration measured is constant after gettering. E Average Decrease in Interstitial Iron Concentration 1.OE+12 1.QE+10 0 1.QE+08 - 5 1.QE+06 10 S1.OE+04 - 1.0+04 1 .OE+02 As grown Post-PDG 1.OE+00 92C 95B 92B 95A 92A 95C Sample Number / Gettering Temperature 900 0C 950 *c 1100 C Figure 6.1: Reduction in Interstitial Iron Concentration Interstitial iron concentration is reduced for each ingot height and gettering temperature. The percentage reduction for each ingot height is plotted in Figure 6.2. The majority of interstitial iron is removed during high-temperature processing or 95% for 92% ingot height samples and 99% for 95% ingot height samples. 56 Mean Percent Reduction in Iron Concentration 100% 0 98%1- 96%- 0 94% 92% * 90% 88% 86% 95C 92A 95A 92B 95B 92C Sample Number / Gettering Temperature 9000C 50 *C 1100 *C Figure 6.2: Percent Reduction in Interstitial Iron Concentration Percentage reduction in interstitial iron concentration is plotted for each ingot height and gettering temperature. 6.3 Lifetime Decreases with Higher PDG Temperature While high-temperature gettering successfully reduces interstitial iron concentration, sample lifetime does not improve accordingly. As interstitial iron is removed from a sample, minority carrier lifetime is expected to increase as shown in equation (3.5). For the majority of the samples, the lifetime increased after phosphorus diffusion gettering. Average percentage increase in minority carrier lifetime is plotted in Figure 6.3 by gettering temperature. As gettering temperature increases, percentage increase in lifetime is lower. For the 95% ingot height samples, with increasing gettering temperature percentage increase in lifetime is lower. For these higher initial iron concentration samples with lower initial lifetime, high-temperature processing always increases the lifetime. Increasing the gettering temperature beyond 900 'C does not result in improved minority carrier lifetime. For the 92% ingot height samples, increasing gettering temperature also results in decreased percentage increase in lifetime. For the sample gettered at 1100 'C, the post-gettered lifetime is lower than the pre-gettered lifetime. 57 Average Percentage Increase in Lifetime 1800% 1600% E e 1400% -J1200% Dark lluminated - 3 1000% 800% e 600% 400% 200% 0%I -200% 92A 95A 92B 95B 92C Sample Number / Gettering Temperature 900 *C 950 OC 950 110D *C Figure 6.3: Percent Reduction in Interstitial Iron Concentration Percentage reduction in [Fei] is plotted for each ingot height and gettering temperature. Average lifetime values for each ingot height are plotted by gettering temperature in Figure 6.4. Note that gettering increases the lifetime for the 95% ingot height samples to nearly equal the 92% ingot height samples. The samples from the 95% ingot height initially had a much higher iron concentration and lower lifetime. High-temperature gettering was able to bring the lifetimes up to the level of the lower contamination samples. 58 Average Lifetime Post-PDG - Dark Illuminated - 100 90 80 70 60 E 50 40 ~30 20 10 0 Sample Number/ Gettering Temperature 900 *C 950 0C 11000C Figure 6.4: Post-Gettered Lifetime by Gettering Temperature Dark and illuminated minority carrier lifetime. Again the trend is clear that increasing gettering temperature results in lower minority carrier lifetime. With increasing gettering temperature from 900 'C to 1100 'C the post-gettered lifetime decreased. As observed by previous studies, higher temperature gettering results in degraded lifetime. In the next sections, spatial analysis is performed to elucidate the source of the thermal degradation. 6.4 Spatially Resolved Lifetime - pt-PCD To understand the spatial resolution of lifetime, the samples were imaged using g-PCD. From the maps in Figure 5.4 and Figure 5.5, lifetime is degraded with increasing temperature. Within each set of sister wafer samples, the lifetime is clearly degraded with increasing gettering temperature at both ingot heights. From the histograms with increased gettering temperature, the entire distribution of lifetime is shifted left indicating uniformly lower lifetime values. 59 Quantifying the impact of high-temperature locally, lifetime for specific regions of the sister samples are compared. Specifically, lifetime distribution and degradation within specific regions are analyzed. The distribution of lifetime degradation within the samples is important to identifying potential impurity or defect causing degradation. To quantify the regional impact of lifetime degradation, sub-regions of the sister samples were selected using grain boundary to ensure similar as-grown contamination and structure. Regions of the same dimensions were aligned tangent to grain boundaries (used as a datum surface) for three sister samples as shown in Figure 6.5 and Figure 6.6. From the selected region, local average lifetime at 950 *C and 1100 *C is compared to that at 900 *C and plotted in Figure 6.7 and fit with a linear approximation. Lifetime degradation with increasing PDG temperature is linear, indicates that lifetime degradation is uniform. 60 PDG 950 ~C PDG 950 TC qnn ~ p1v Mr. QAA or 5 (D 1 Figure 6.5: Sub-Region Selection for Lifetime Comparison 95% Regions of varying lifetime with well-defined grain boundaries were compared. Average lifetime was calculated for each region. PDG 900 *C PDG 950 *C PDG 1100 *C (D0 3 Figure 6.6: Sub-region selection for Lifetime Comparison 92% Regions of varying lifetime with well-defined grain boundaries were compared. Average lifetime was calculated for each region. 61 Lifetime Degradation by Temperature . 100 * 90 E T950 -C M T110010 C 80 S70 60 50 40 E cL30 A! 20 Do 10 0 0 20 40 60 80 Lifetime for PDG 900 0C (ps) 100 120 Figure 6.7: Post-Gettered Lifetime Degradation by Temperature Lifetime at 950 *C and I100 0 C is compared to lifetime at 900 'C to determine the uniformity of thermal degradation. Linear trend indicates uniform lifetime degradation. For each linear fit, the slope was calculated to estimate the impact of increased gettering temperature on number of defects. Lifetime in a wafer after gettering at a given temperature T can be expressed as -CT. Assuming one defect type is governing the lifetime, then TT is proportional to the density of defects remaining in the wafer after gettering at that temperature, ND,T, as described by equation (6.1), where C is a constant. C (6.1) TT = ND,T From equation 6.1, the ratio of lifetime at two different gettering temperatures is correlated to the ratio of density of defects in equation (6.2). 74T_ T900 ND,90 0 (6.2) ND,T The lifetime ratio corresponds to the slope of each linear fit in Figure 6.7 reported in Table 6.1. From the slope, the percentage increase in defect density is calculated from the inverse of the slope also reported in Table 6.1. The calculated values indicate an increase in defect density with increasing gettering temperature. Gettering at 950 0 C and 1100 'C instead of 900 'C 62 increases the defect density by 23% and 236% respectively. While caution must be taken with a limited data set, the supra-linear relationship between defect density and gettering temperature suggests the possibility that the lifetime-limiting defect formed at the gettering temperature and was minimally affected by the cool-down process. Table 6.1: Slope of Lifetime Degradation by Temperature T 950*C 1100*C TT 0.810 0.298 NDT ND,TO ND,900 1.23 3.36 6.5 Spatially Resolved Lifetime - PL Imaging Comparing the PL images of sister wafers highlights the impact of high-temperature gettering on lifetime in different regions of the multicrystalline silicon samples. When analyzing samples, sister wafers were compared to understand the uniformity of high-temperature processing on grain structures and regions of the sample. For one set of sister samples, MG 1118, MG 1119, and MG 1120, enlarged PL images are plotted for comparison in Figure 6.8. At first glance, the PL images are progressively crisper with increasing gettering temperature. The blurriness at for the low gettering temperature sample is due to the higher lifetime of the material. When imaging higher-lifetime material, minority carriers can travel longer distances before recombining, causing the image to blur. Comparing the impact of gettering on the distribution of lifetime within a grain, one can notice a distinct difference between samples gettered at different temperatures in Figure 6.8. As gettering temperature increases, the grains suggest an increase in dislocation defects evidenced by spider-web-like pattern. For example, the large grain in lower right hand corner exhibits increased spider web pattering after higher temperature gettering. The change could evidence an increase in thermal degradation at high-temperature, or simply the benefit of enhanced spatial resolution due to shorter minority-carrier lifetime (lack of blurring). With increasing gettering temperature, the large grains also show a decrease in dark spots and the grains are more uniformly shaded. The decrease in dark spots could indicate removal or 63 decreased size of larger impurities. The uniform grain shade indicates a uniform lifetime distribution across the grain. Specifically, lifetime does not decrease as proximity to the grain boundary increases. Gradients in lifetime do not appear near grain boundaries or point defects. The uniformity of the lifetime degradation indicates that the high-temperature degradation defect is uniformly distributed across the sample. Figure 6.8: PL Image of Sister Samples by Gettering Temperature Brighter regions indicate higher lifetime. Similar to pt-PCD, PL image analysis of sister wafers suggest uniform lifetime degradation for increased gettering temperatures. 64 6.6 Candidate Sources of High-Temperature Degradation To identify a source of high-temperature degradation, literature on high-temperature gettering was reviewed and summarized in Table 6.2. Table 6.2: Candidate High-Temperature Contaminants Contaminant Hypothesis Measurement Literature High-temperature changed the phase of oxygen in the bulk from an inert form (interstitial) to a recombination-active FTIR to measure Falster [60], interstitial oxygen. Pizzini [61], H. Hiesimair [62] Oxygen Interstitial oxygen form (chain-like structure or precipitate). Metal Impurities Ti High-temperature PDG dissolves Ti precipitates. Slow diffuser does not have sufficient time to getter to the emitter. DLTS Chen 1979 [63], K. Leo [64], A. Rohatgi[65] Cr High-temperature PDG dissolves Cr precipitates. Slow diffuser does not have sufficient time to getter to the emitter. Thermal disassociation with Sinton tool and QSSPC method. -- Mo High-temperature PDG dissolves Mo DLTS A. Rohatgi [65] precipitates and standard POC1 3 processes do not adequately remove Mo. Other Synchrotron measurement could identify common contaminants in as-grown Existing synchrotron data for MG as-grown material. material at grain boundaries. Dislocation Thermal stress during loading and unloading Hightemperature plastic flow Thermal stress from loading and unloading increases the dislocation concentration. This seems unlikely as all the samples were loaded and unloaded at the same temperature. Increase in dislocation density due to plastic flow at high-temperature, e.g., caused by mechanical loading or relaxation of residual stress at as-grown Dislocation etch Franke [66] Dislocation etch Macdonald 2000 [67] defects. 65 6.7 Furnace Cool Rate Verification As cool rate is critical to gettering as shown by simulation and experiments [68], the furnace cool rate was verified from the temperature signal recorded during getting experiments. Taking the average of the time derivative of the temperature signal provides an estimate for the change in temperature per unit time. Plots of the average change in furnace temperature are plotted in Figure 6.9 and Figure 6.10. Derivative of Average Furnace Temperature durring PDG at 950 *C 10 Figure 6.9: Derivative of Mean Furnace Temperature during 950 *C PDG Ramp rate is 10 *C/min. Peak cool rate is 5 'C/min. Derivative of Average Furnace Temperature durring PDG at 1100 T 10 0 j Time (imi Figure 6.10: Derivative of Mean Furnace Temperature during 1100 'C PDG Ramp rate is 10 *C/min. Peak cool rate is 8 *C/min. At higher furnace temperature the cool rate increases, as the temperature gradient between the furnace and surroundings is higher. Temperature cool rate can be controlled to reduce any variation in thermal stress for future experiments. 66 6.8 Baseline Gettering at 845 0 C To review the range of high-temperatures on gettering, the results of the high-temperature gettering study were compared to samples from the same ingot gettered at a plateau temperature of 845 *C with slow cool rate (-4 *C/min). Samples from the same ingot heights as the original samples have similar initial iron concentration and consistent grain structure therefore provide a useful baseline for the high-temperature gettering study. For the 95% ingot height, samples compared were cut from the same wafer. For the 92% ingot height, samples compared were averaged from proximate wafers. Note that samples for the 845 *C are not sister wafers or samples directly adjacent from different wafers, which increase uncertainty. Especially in multicrystalline silicon, lifetime and impurities are particularly dependent on grain structure. Grain structure is most closely preserved between sample sets when sister wafers are selected. While sister wafers were not available, the comparison still provides a valuable baseline. Average minority carrier lifetime for the 92% and 95% ingot heights are plotted by temperature in Figure 6.11 and Figure 6.12. (Lifetime measurements are reported at 5 x 1015 injection level.) For the 92% ingot height samples, improvement in minority carrier lifetime at higher temperature is not definitive (especially due to a broken sample resulting in a low number of samples at 900 *C). For the 95% ingot height samples, the lifetime is improved by increasing gettering temperature to 900 *C, but beyond this point the lifetime degrades with increasing temperature. 67 Average Lifetime Post-PDG by Temperature for 92% Ingot Height Samples 120 U Dark 100 Illuminated - 80 60 - E 40 - 20 0 845 900 950 1100 Gettering Temperature (C) Figure 6.11: Average Lifetime Post-PDG by Temperature (92% Ingot Height) Dark and illuminated minority carrier lifetime. Average Lifetime Post-PDG by Temperature for 95% Ingot Height Samples 120 0 Dark Illuminated ~ 100 --- 60 - E 40 - 80 - 20 0 845 900 950 1100 Gettering Temperature (*C) Figure 6.12: Average Lifetime Post-PDG by Temperature (95% Ingot Height) Dark and illuminated minority carrier lifetime. 68 6.9 Conclusion High-temperature, "sacrificial" gettering processes were explored to optimize iron removal, without consideration for emitter quality. We utilize a kinetic gettering simulation, 12E, to predict the effect of high-temperature gettering on iron concentration in multicrystalline silicon. With increasing temperature, the solubility and diffusivity of iron in silicon increases. Confirming the predicted simulation results, high-temperature gettering with a controlled cool down was a successful method for reducing interstitial iron in mc-Si. Yet, after high-temperature gettering, lifetime is not improved proportionally with decreased iron concentration. Iron is no longer the lifetime-limiting defect in gettered material. Another uniformly distributed defect likely is generated after higher-temperature processing and is responsible for the observed lifetime degradation. With optimized gettering profiles, iron does not place an upper bound on maximum gettering temperature. Additional research must be conducted, to elucidate the nature of the performance-limiting defect(s) generated after hightemperature gettering. 69 70 REFERENCES [1] G. Coletti, "Sensitivity of State-of-the-Art and High Efficiency Crystalline Silicon Solar Cells to Metal Impurities," Prog. Photovolt. Res. Appl., vol. 21, no. 5, pp. 1163-1170, Aug. 2013. [2] J. Hofstetter, D. P. Fenning, M. I. Bertoni, J. F. Lelievre, C. del Caftizo, and T. Buonassisi, "Impurity-to-Efficiency Simulator: Predictive Simulation of Silicon Solar Cell Performance Based on Iron Content and Distribution," Prog. Photovolt. Res. Appl., vol. 19, no. 4, pp. 487-497, Jun.2011. [3] IEA (2013), "World Energy Outlook 2013," IEA, 2013. [4] U.S. Energy Information Administration, "International Energy Outlook 2013: With Projections to 2040," U.S. Department of Energy, Washington, DC, Jul. 2013. [5] J. Rockstr6m, W. Steffen, K. Noone, A. Persson, F. Stuart III Chapin, E. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. Van der Leeuw, H. Rodhe, S. S~rlin, P. K. Snyder, R. Costanza, U. Svedin, and M. Falkenmark, "Planetary Boundaries: Exploring the Safe Operating Space for Humanity.," Ecol. Soc., vol. 14, no. 2, pp. 1-33, Dec. 2009. [6] N. Polunin and M. Nazim, Eds., "Population and Global Security Limited Geneva Edition," Environ. Conserv., vol. 21, no. 02, pp. 98-98, 1994. [7] N. Stylos and C. Koroneos, "Carbon Footprint of Polycrystalline Photovoltaic Systems," J. Clean. Prod, vol. 64, pp. 639-645, Feb. 2014. [8] D. Nugent and B. K. Sovacool, "Assessing the Lifecycle Greenhouse Gas Emissions from Solar PV and Wind Energy," Energy Policy, vol. 65, pp. 229-244, Feb. 2014. [9] J. W. Tester, E. M. Drake, M. J. Driscoll, M. W. Golay, and W. A. Peters, Sustainable Energy: ChoosingAmong Options. Cambridge, MA: MIT Press, 2012. [10] S. Chu and A. Majumdar, "Opportunities and Challenges for a Sustainable Energy Future," Nature, vol. 488, no. 7411, pp. 294-303, Aug. 2012. [11] P. Wfirfel, Physics of Solar Cells: from Principles to New Concepts. Weinheim: WileyVCH, 2005. [12] C. A. Wolden, J. Kurtin, J. B. Baxter, I. Repins, S. E. Shaheen, J. T. Torvik, A. A. Rockett, V. M. Fthenakis, and E. S. Aydil, "Photovoltaic Manufacturing: Present Status, Future Prospects, and Research Needs," J Vac. Sci. Technol. Part -Vac. Surf Films, vol. 29, no. 3, p. 030801, May 2011. DOE, [13] US "SunShot Mission," energy.gov. [Online]. Available: http://energy.gov/eere/sunshot/mission. [Accessed: 12-May-2014]. [14] D. M. Powell, M. T. Winkler, A. Goodrich, and T. Buonassisi, "Modeling the Cost and Minimum Sustainable Price of Crystalline Silicon Photovoltaic Manufacturing in the United States," IEEEJ Photovolt., vol. 3, no. 2, pp. 662-668, Apr. 2013. [15] A. A. Istratov, H. Hieslmair, and E. R. Weber, "Iron Contamination in Silicon Technology," Appl. Phys. Mater. Sci. Process., vol. 70, no. 5, p. 489, May 2000. [16] H. J. Queisser and E. E. Haller, "Defects in Semiconductors: Some Fatal, Some Vital," Science, no. 5379, p. 945, 1998. 71 [17] S. A. McHugo and H. Hieslmair, "Gettering in Silicon," in Wiley Encyclopedia of Electricaland ElectronicsEngineering, John Wiley & Sons, Inc., 2001. [18] J. R. Davis, A. Rohatgi, R. H. Hopkins, P. D. Blais, P. Rai-Choudhury, J. R. McCormick, and H. C. Mollenkopf, "Impurities in Silicon Solar Cells," IEEE Trans. Electron Devices, vol. 27, no. 4, p. 677, Jan. 1980. [19] J. Schmidt, B. Lim, D. Walter, K. Bothe, S. Gatz, T. Dullweber, and P. P. Altermatt, "Impurity-Related Limitations of Next-Generation Industrial Silicon Solar Cells," IEEE J. Photovolt., vol. 3, no. 1, pp. 114-118, Jan. 2013. [20] A. . Istratov, H. Hieslmair, and E. . Weber, "What Do We Know about Iron in Silicon after 45yr of Research," Phys. B Phys. Condens. Matter, vol. 273, pp. 412-415, Jan. 1999. [21] B. Sopori, "Silicon Solar-Cell Processing for Minimizing the Influence of Impurities and Defects," J. Electron. Mater., vol. 31, no. 10, pp. 972-980, Oct. 2002. [22] A. A. Istratov, T. Buonassisi, R. J. McDonald, A. R. Smith, R. Schindler, J. A. Rand, J. P. Kalejs, and F. R. Weber, "Metal Content of Multicrystalline Silicon for Solar Cells and Its Impact on Minority Carrier Diffusion Length," J. Appl. Phys., vol. 94, no. 10, pp. 65526559, Nov. 2003. [23] T. Buonassisi, A. A. Istratov, M. Heuer, M. A. Marcus, R. Jonczyk, J. Isenberg, B. Lai, Z. Cai, S. Heald, W. Warta, R. Schindler, G. Willeke, and E. R. Weber, "Synchrotron-Based Investigations of the Nature and Impact of Iron Contamination in Multicrystalline Silicon Solar Cells," J. Appl. Phys., vol. 97, no. 7, p. 074901, Apr. 2005. [24] D. Macdonald, A. Cuevas, and J. Wong-Leung, "Capture Cross Sections of the Acceptor Level of Iron-Boron Pairs in P-Type Silicon by Injection-Level Dependent Lifetime Measurements," J Appl. Phys., vol. 89, no. 12, p. 7932, Jun. 2001. [25] G. Coletti, R. Kvande, V. D. Mihailetchi, L. J. Geerligs, L. Arnberg, and E. J. Ovrelid, "Effect of Iron in Silicon Feedstock on P- and N-Type Multicrystalline Silicon Solar Cells," J. Appl. Phys., vol. 104, no. 10, p. 104913, Nov. 2008. [26] Y. Delannoy, "Purification of Silicon for Photovoltaic Applications," 5th Int. Workshop Cryst. Growth Technol., vol. 360, no. 0, pp. 61-67, Dec. 2012. [27] M. A. Green, Solar Cells: Operating Principles, Technology, and System Applications. Englewood Cliffs, NJ: Prentice-Hall, 1982. [28] S. Bernardis, B. K. Newman, M. Di Sabatino, S. C. Fakra, M. I. Bertoni, D. P. Fenning, R. B. Larsen, and T. Buonassisi, "Synchrotron-Based Microprobe Investigation of Impurities in Raw Quartz-Bearing and Carbon-Bearing Feedstock Materials for Photovoltaic Applications," Prog. Photovolt. Res. Appl., vol. 20, no. 2, pp. 217-225, Mar. 2012. [29] F. Schindler, B. Michl, J. Schon, W. Kwapil, W. Warta, and M. C. Schubert, "Solar Cell Efficiency Losses Due to Impurities From the Crucible in Multicrystalline Silicon," IEEE J. Photovolt., vol. 4, no. 1, pp. 122-129, Jan. 2014. [30] D. Macdonald, A. Cuevas, A. Kinomura, Y. Nakano, and L. J. Geerligs, "Transition-Metal Profiles in a Multicrystalline Silicon Ingot," J. Appl. Phys., vol. 97, no. 3, p. 033523, Feb. 2005. [31] J. Hofstetter, J.-F. Lelievre, D. P. Fenning, M. I. Bertoni, T. Buonassisi, and C. del Canizo, "Towards the Tailoring of P-Diffusion Gettering to As-Grown Silicon Material Properties," Diffus. Defect Data Part B Solid State Phenom., vol. 178-179, no. 178-179, pp. 158-165, Jan. 2011. [32] M. A. Green, Silicon Solar Cells: Advanced Principles & Practice. Sydney, Australia: Centre for Photovoltaic Devices and Systems, 1995. 72 [33] D. P. Fenning, "High-temperature Defect Engineering for Silicon Solar Cells: Predictive Process Simulation and Synchrotron-Based Microcharacterization," MIT, 2013. [34] A. E. Morishige, "Co-Optimizing Silicon Solar Cell Processing for Efficiency and Throughput," MIT, 2013. [35] S. Noel, A. Slaoui, S. Peters, H. Lautenschlager, R. Schindler, and J. C. Muller, "Optimized Rapid Thermal Process for High Efficiency Silicon Solar Cells," in Solar Energy MaterialsandSolar Cells, 2001, vol. 65, pp. 495-501. [36] S. Peters, J. Y. Lee, C. Ballif, D. Borchert, S. W. Glunz, W. Warta, and G. Willeke, "Rapid Thermal Processing: A Comprehensive Classification of Silicon Materials," presented at the Conference Record of the Twenty-Ninth IEEE Photovoltaic Specialists Conference 2002 (Cat. No.02CH37361), 2002. [37] D. P. Fenning, J. Hofstetter, M. I. Bertoni, G. Coletti, B. Lai, C. del Cafiiizo, and T. Buonassisi, "Precipitated Iron: A Limit on Gettering Efficacy in Multicrystalline Silicon," J. Appl. Phys., vol. 113, no. 4, pp. 044521-044521-12, Jan. 2013. [38] W. Kwapil, J. Schon, F. Schindler, W. Warta, and M. C. Schubert, "Impact of Iron Precipitates on Carrier Lifetime in As-Grown and Phosphorus-Gettered Multicrystalline Silicon Wafers in Model and Experiment," IEEE J. Photovolt., vol. 4, no. 3, pp. 791-798, May 2014. [39] J. Hofstetter, D. P. Fenning, J.-F. Lelievre, C. del Cafiizo, and T. Buonassisi, "Engineering Metal Precipitate Size Distributions to Enhance Gettering in Multicrystalline Silicon," Phys. Status Solidi A, vol. 209, no. 10, pp. 1861-1865, Oct. 2012. [40] David P. Fenning, Jasmin Hofstetter, Ashley E. Morishige, Douglas M. Powell, Annika S. Zuschlag, Giso Hahn, and Tonio Buonassisi, "Darwin at High Temperature: Advancing Solar Cell Material Design through Defect Kinetics Simulation and Evolutionary Optimization," Adv Energy Mater, 2014. [41] J. Hofstetter, "Defect Engineering Strategies for Solar Grade Silicon and their Optimization by Predictive Simulation," Doctoral Thesis, Universidad Politecnica de Madrid, 2011. [42] D. A. Clugston and P. A. Basore, "PC1D version 5: 32-Bit Solar Cell Modeling on Personal Computers," in , Conference Record of the Twenty-Sixth IEEE Photovoltaic Specialists Conference, 1997, 1997, pp. 207-210. [43] A. Bentzen, A. Holt, J. S. Christensen, and B. G. Svensson, "High Concentration in- Diffusion of Phosphorus in Si from a Spray-on Source," J Appl. Phys., vol. 99, no. 6, p. 064502, Mar. 2006. [44] T. Y. Tan, R. Gafiteanu, S. M. Joshi, U. Gosele, H. R. Huff, H. Tsuya, and U. Gosele, "Science and Modeling of Impurity Gettering in Silicon," presented at the Proceedings of the Eighth International Symposium on Silicon Materials Science and Technology. Silicon Materials Science and Technology, 1998, vol. 2. [45] A. Haarahiltunen, H. Savin, M. Yli-Koski, H. Talvitie, and J. Sinkkonen, "Modeling Phosphorus Diffusion Gettering of Iron in Single Crystal Silicon," J. Appl. Phys., vol. 105, no. 2, Jan. 2009. [46] C. del Canizo and A. Luque, "A Comprehensive Model for the Gettering of LifetimeKilling Impurities in Silicon," J. Electrochem. Soc., vol. 147, no. 7, pp. 2685-2692, Jul. 2000. [47] W. Shockley and W. T. Read jr, "Statistics of the Recombinations of Holes and Electrons," Phys. Rev., vol. 87, pp. 835-842, Sep. 1952. 73 [48] R. N. Hall, "Electron-Hole Recombination in Germanium," Phys. Rev., vol. 87, pp. 387387, Jul. 1952. [49] H. Wagner, M. MUller, G. Fischer, and P. P. Altermatt, "A Simple Criterion for Predicting Multicrystalline Si Solar Cell Performance from Lifetime Images of Wafers Prior to Cell Production," J. Appl. Phys., vol. 114, no. 5, p. 054501, Aug. 2013. [50] G. Dingemans and W. M. M. Kessels, "Status and Prospects of A1203-Based Surface Passivation Schemes for Silicon Solar Cells," J. Vac. Sci. Technol. A, vol. 30, no. 4, p. 040802, Jul. 2012. [51] R. A. Sinton and A. Cuevas, "Contactless Determination of Current-Voltage Characteristics and Minority-Carrier Lifetimes in Semiconductors from Quasi-Steady-State Photoconductance Data," Appl. Phys. Lett., vol. 69, no. 17, p. 2510, Oct. 1996. [52] H. Nagel, C. Berge, and A. G. Aberle, "Generalized Analysis of Quasi-Steady-State and Quasi-Transient Measurements of Carrier Lifetimes in Semiconductors," J Appl. Phys., vol. 86, no. 11, pp. 6218-6221, Dec. 1999. [53] R. A. Sinton and D. Macdoald, "Sinton WCT-120 and Suns-VOC manual." Sinton Consulting, Inc, 2006. [54] G. Zoth and W. Bergholz, "A Fast, Preparation-Free Method to Detect Iron in Silicon," J. Appl. Phys., vol. 67, no. 11, p. 6764, 6/1/1990 1990. [55] M. c. Schubert, H. Habenicht, and W. Warta, "Imaging of Metastable Defects in Silicon," IEEEJ. Photovolt., vol. 1, no. 2, pp. 168-173, Oct. 2011. [56] SEMILAB Rt., "WT2000-1 Theory." 2003. [57] Sandra Herlufsen, Jan Schmidt, David Hinken, Karsten Bothe, and Rolf Brendel, "Photoconductance-Calibrated Photoluminescence Lifetime Imaging of Crystalline Silicon," Phys Stat Sol, no. 6, pp. 245-247, Sep. 2008. [58] D. Macdonald, J. Tan, and T. Trupke, "Imaging Interstitial Iron Concentrations in BoronDoped Crystalline Silicon Using Photoluminescence," J. Appl. Phys., vol. 103, no. 7, p. 073710, Apr. 2008. [59] D. P. Fenning, A. S. Zuschlag, J. Hofstetter, A. Frey, M. I. Bertoni, G. Hahn, and T. Buonassisi, "Investigation of Lifetime-Limiting Defects After High-Temperature Phosphorus Diffusion in High-Iron-Content Multicrystalline Silicon," IEEE J. Photovolt., vol. 4, no. 3, pp. 866-873, May 2014. [60] R. Falster and V. Voronkov, "Rapid Thermal Processing and the Control of Oxygen Precipitation Behaviour in Silicon Wafers," Mater. Sci. Forum, vol. 573-574, pp. 45-60, Mar. 2008. [61] S. Pizzini, L. Bigoni, and M. Beghi, "On the Effect of Impurities on the Photovoltaic Behavior of Solar Grade Silicon," J Electrochem. Soc., vol. 133, pp. 2363-2373, Nov. 1986. [62] H. Hieslmair, A. A. Istratov, S. A. McHugo, C. Flink, T. Heiser, and E. R. Weber, "Gettering of Iron by Oxygen Precipitates," Appl. Phys. Lett., vol. 72, no. 12, pp. 14601462, Mar. 1998. [63] J.-W. Chen, A. G. Milnes, and A. Rohatgi, "Titanium in Silicon as a Deep Level Impurity," Solid-State Electron., vol. 22, no. 9, pp. 801-808, Sep. 1979. [64] K. Leo, R. Schindler, J. Knobloch, and B. Voss, "Titanium Gettering in Silicon: Investigation by Deep Level Transient Spectroscopy and Secondary Ion Mass Spectroscopy," J. Appl. Phys., vol. 62, no. 8, p. 3472, Oct. 1987. [65] A. Rohatgi, R. B. Campbell, J. R. Davis, R. H. Hopkins, P. Rai-Choudhury, H. Mollenkopf, and J. R. McCormick, "POCl3 Gettering of Titanium, Molybdenum and Iron74 Contaminated Silicon Solar Cells," presented at the Fourteenth IEEE Photovoltaic Specialists Conference 1980, 1980. [66] D. Franke, K. Kurokawa, L. L. Kazmerski, B. McNelis, M. Yamaguchi, C. Wronski, and W. C. Sinke, "Rise of Dislocation Density in Crystalline Silicon Wafers During Diffusion Processing," presented at the Proceedings of 3rd World Conference on Photovoltaic Energy Conversion (IEEE Cat. No.03CH37497), 2003, vol. 2. [67] Daniel Macdonald and Andres Cuevas, "The Trade-Off Between Phosphorus Gettering and Thermal Degredation in Multicrystaline Silicon," presented at the 16th European Photovoltaic Solar Energy Conference, Glasgow, 2000. [68] J. Hofstetter, J. F. Lelievre, C. del Caffizo, and A. Luque, "Study of Internal Versus External Gettering of Iron During Slow Cooling Processes for Silicon Solar Cell Fabrication," Diffus. Defect Data Part B Solid State Phenom., vol. 156-8, no. 156-8, pp. 387-393, Jan. 2010. 75