Average atomic mass worksheet
advertisement
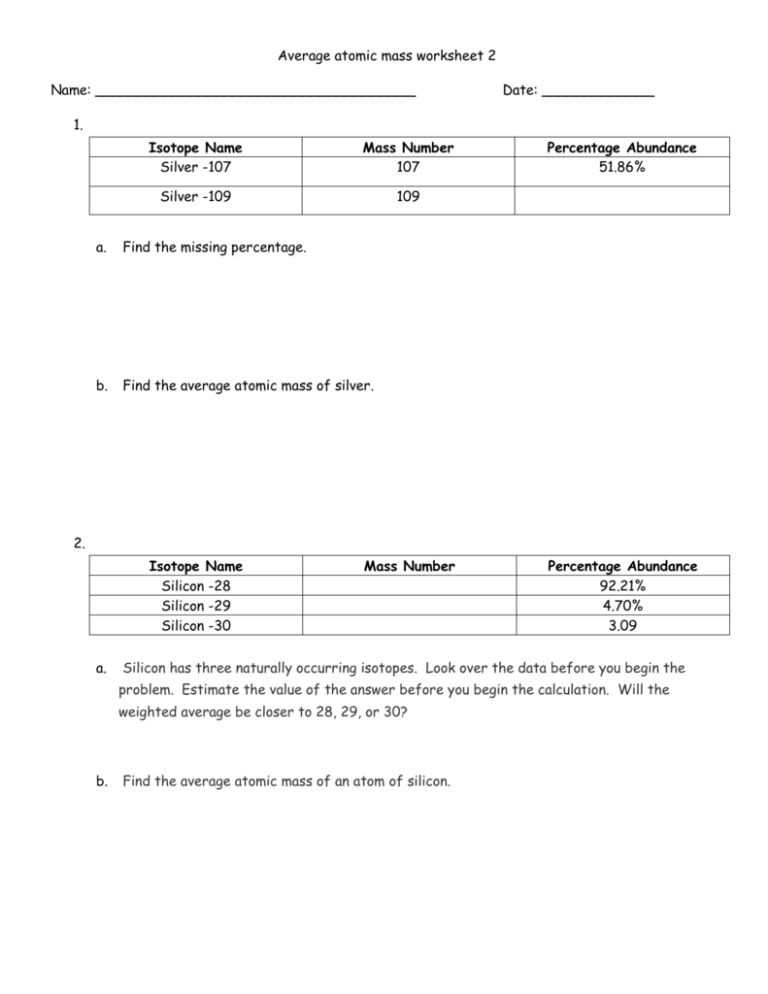
Average atomic mass worksheet 2 Name: _____________________________________ Date: _____________ 1. Isotope Name Silver -107 Mass Number 107 Silver -109 109 a. Find the missing percentage. b. Find the average atomic mass of silver. Percentage Abundance 51.86% 2. Isotope Name Silicon -28 Silicon -29 Silicon -30 a. Mass Number Percentage Abundance 92.21% 4.70% 3.09 Silicon has three naturally occurring isotopes. Look over the data before you begin the problem. Estimate the value of the answer before you begin the calculation. Will the weighted average be closer to 28, 29, or 30? b. Find the average atomic mass of an atom of silicon. 3. Isotope Name Iron -54 Iron -56 Iron -57 Iron -58 a. Mass Number Calculate the average atomic mass of iron. 4. Titanium has five common Isotopes: 46Ti (8.0%), 47Ti (7.8%), (5.3%). What is the average atomic mass of titanium? 5. Percentage Abundance 5.9% 91.72% 2.10% 0.280% 48 Ti (73.4%), 49 Ti (5.5%), 50 Ti A chemistry student’s grade is weighted. Tests are worth 50.0 %, labs are worth 25.0 %, and homework is worth 25.0 %. A student’s test average is 85.0 %, lab average is 77.0 %, and homework is 91.0 %. a. What is the students average grade?