PHY 3513:Kumar I Midterm Examination: Solutions
advertisement
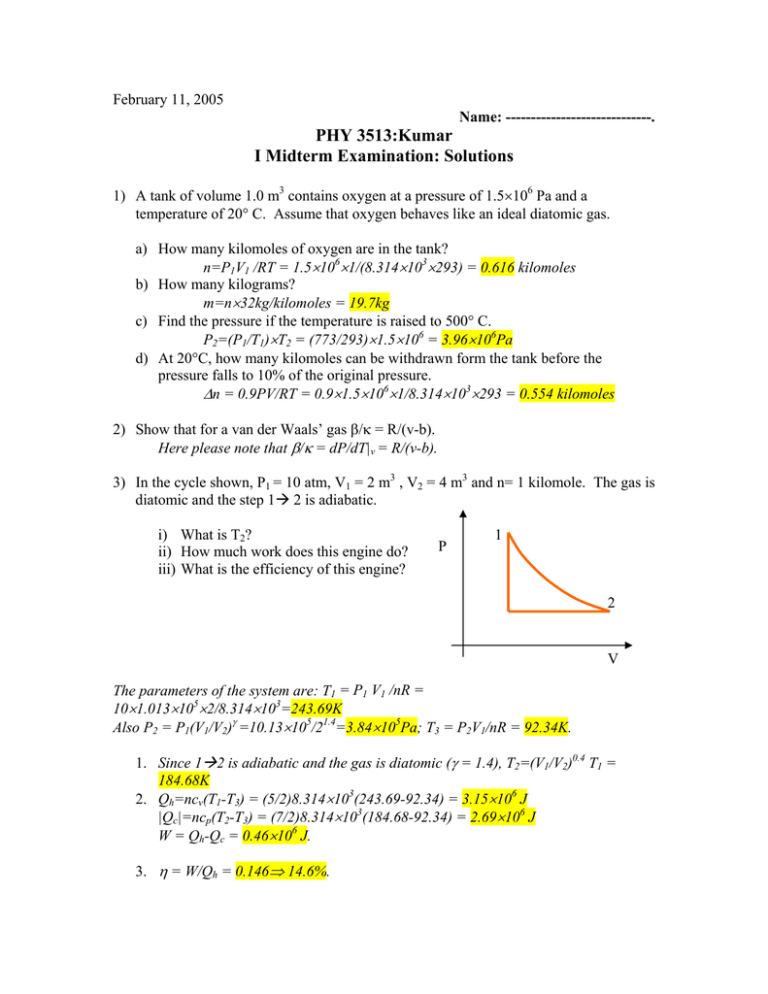
February 11, 2005 Name: -----------------------------. PHY 3513:Kumar I Midterm Examination: Solutions 1) A tank of volume 1.0 m3 contains oxygen at a pressure of 1.5×106 Pa and a temperature of 20° C. Assume that oxygen behaves like an ideal diatomic gas. a) How many kilomoles of oxygen are in the tank? n=P1V1 /RT = 1.5×106×1/(8.314×103×293) = 0.616 kilomoles b) How many kilograms? m=n×32kg/kilomoles = 19.7kg c) Find the pressure if the temperature is raised to 500° C. P2=(P1/T1)×T2 = (773/293)×1.5×106 = 3.96×106Pa d) At 20°C, how many kilomoles can be withdrawn form the tank before the pressure falls to 10% of the original pressure. Δn = 0.9PV/RT = 0.9×1.5×106×1/8.314×103×293 = 0.554 kilomoles 2) Show that for a van der Waals’ gas β/κ = R/(v-b). Here please note that β/κ = dP/dT|v = R/(v-b). 3) In the cycle shown, P1 = 10 atm, V1 = 2 m3 , V2 = 4 m3 and n= 1 kilomole. The gas is diatomic and the step 1Æ 2 is adiabatic. i) What is T2? ii) How much work does this engine do? iii) What is the efficiency of this engine? P 1 2 V The parameters of the system are: T1 = P1 V1 /nR = 10×1.013×105×2/8.314×103=243.69K Also P2 = P1(V1/V2)γ =10.13×105/21.4=3.84×105Pa; T3 = P2V1/nR = 92.34K. 1. Since 1Æ2 is adiabatic and the gas is diatomic (γ = 1.4), T2=(V1/V2)0.4 T1 = 184.68K 2. Qh=ncv(T1-T3) = (5/2)8.314×103(243.69-92.34) = 3.15×106 J |Qc|=ncp(T2-T3) = (7/2)8.314×103(184.68-92.34) = 2.69×106 J W = Qh-Qc = 0.46×106 J. 3. η = W/Qh = 0.146⇒ 14.6%. 4) Forty kg of water at 0°C must be frozen into ice in a refrigerator. The room temperature is 25° C. The latent heat of fusion of water is 3.33×105 J/kg. What is the minimum power required if the freezing has to occur in an hour? Minimum power must come from the most efficient refrigerator, which is a Carnot cycle. The coefficient of performance of a Carnot refrigerator is c = Qc/W = Tc/(ThTc). T − Tc 1 1 25 Therefore the power P = h Qc = =339W. 40 × 3.33 × 10 5 3600 Tc 3600 273











