Repellency, Efficacy, and Persistence of Two Microbial Control Agents
advertisement

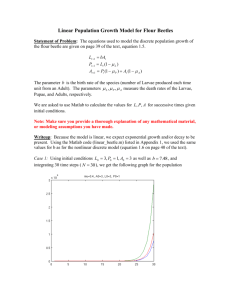
Repellency, Efficacy, and Persistence of Two Microbial Control Agents of an Asian Ambrosia Beetle and it’s Symbiotic Fungus Edna Bailey Sussman Foundation Research Report December 2013 Joelle Chille, MS Candidate SUNY College of Environmental Science and Forestry SUMMARY OF PROPOSED WORK Asian ambrosia beetles, particularly two species of the genus Xylosandrus, are among the most important exotic pests of orchards and nurseries in the United States. These invasive ambrosia beetles are generalist woodborers, attacking a wide range of tree and shrub species including beech, black walnut, dogwood, elm, maple, and oak. While native ambrosia beetles tend to attack highly stressed or dying trees, these Asian ambrosia beetles may readily attack healthy host trees. Like most ambrosia beetles, these invasive species tunnel through the sapwood, carving galleries in which they cultivate a symbiotic fungus as food source for both larvae and adults. The symbiotic relationship between insect and fungus likely facilitates the wide host range of the beetle, as the fungus can grow on a very broad range of host trees. The timing of tree attacks varies seasonally as beetles typically have more than one generation per year. My research aims to better understand the susceptibility of both the beetles and their symbiotic fungus to microbial control agents that have been shown in previous research to cause mortality in both organisms. This research will provide information essential to developing management approaches for Asian ambrosia beetles. These and related pests threaten the economic 1 viability of the nursery tree industry. A better understanding of beetle and fungal response to the microbial control agents, as well as the persistence of these agents in the field, is an essential element for developing biological control approaches for these invasive pests. Figure 1: An adult female Black Stem Borer ambrosia beetle, approximately 2.0-2.5 mm in length. RESEARCH OBJECTIVES 1) Determine beetle survival and reproductive success following application of two commercially available microbial control agents: one that attacks the beetle (Met52®) and one that attack’s the beetle’s symbiotic fungus (RootShield®) in the field. 2) Evaluate persistence of the two microbial agents in the field. WORK COMPLETED Beetle survival and Reproductive Success 2 The two microbial control agents were separated into two separate experiments. The first experiment involved the commercial microbial agent Met52, an insect-killing fungus that attacks the beetle pest. A humectant (Barricade Fire Gel®, diluted to 1%) that is theorized to increase fungal spore development was included in some treatments. The four treatments for the Met52 trials were: water, Barricade, Met52, and Met52 + Barricade. The second experiment involved the commercial microbial agent RootShield, a mycoparasitic fungus that attacks the beetle’s symbiotic fungus, which is the beetle’s sole food source. A formulation blank of RootShield was available, which is the commercial product without the active ingredient, the fungus. This blank was examined to determine if the observed effects were due to the fungus, the formulation, or a combination of both. The treatments for the RootShield experiments were: water, RootShield formulation blank, RootShield 1X (the recommended dosage) and RootShield 2X (twice the recommended dosage). For both microbial agents, the field methods were the same. American beech saplings were cut into 25 cm “loglets” and soaked in either 15% ethanol (a beetle attractant) or water (control) for 2-4 days. They were then strapped to stakes, the treatments were applied via backpack spray application, and they were placed in pairs with a rain guard on top in a hardwood stand in Ithaca, NY that the beetles are known to occupy. There were either 6 or 12 replicates per treatment, with each experiment completed twice. Beetle attacks were tallied every 24 hours for three days per experiment. Loglets were then incubated at 25°C for 3-4 weeks to encourage beetle development. Following incubation, loglets were sliced into disks using a band saw and 3 evaluated for presence of the original beetle, gallery length, presence of the original symbiont, and brood size. Figure 2: Left, the author counting beetle attacks on a loglet. Right, the author clips a sample from a loglet to test persistence Persistence of Microbial Agents Due to time constraints, only RootShield was able to be tested for persistence. Twenty total American beech loglets were split into two equal groups and placed in either a sunny, non-shaded area or a dense, shaded, woody area, and oriented either Northfacing or South-facing. They were then sprayed with RootShield 1X and 2.5 inch samples were clipped from the loglets at the following time intervals: 0, 1, 2, 4, 7, and 14 days. The samples were then diluted and plated on a specific medium, where fungal colonies were counted following 48 hours of incubation at 25°C. 4 PRELIMINARY RESULTS For the Met52 trials, both Met52 and Met52 + Barricade sprayed loglets had fewer beetle attacks. Of the remaining attacks, Met52 and Met52 + Barricade sprayed loglets also killed more of the original beetles and reduced brood size. RootShield formulation blank, 1X, and 2X sprayed loglets had fewer beetle attacks, and of the remaining attacks reduced brood size. RootShield 1X and 2X sprayed loglets prevented the beetle’s symbiotic fungus from establishing or thriving. Figure 3: Proportion of beetle offspring present for the Met52 experiment. 5 Figure 4: Proportion of beetle offspring present for the RootShield experiment. Figure 5: Beetle galleries found during loglet dissections in the RootShield experiment showing symbiotic fungus growth. Trichoderma is the active fungal ingredient in RootShield. 6 FUTURE DIRECTIONS Analysis of the persistence data, although collected, has yet to be analyzed. Raw data indicates that fungal colonies are present after 14 days, however. Although these results are promising for the use of these microbial agents to combat Asian Ambrosia beetles, further research needs to be conducted before they are used commercially. Particularly, further persistence testing, especially of Met52, and field trials of both microbial agents in a commercial nursery are needed. ACKNOWLEDGEMENTS I would like to thank the Edna Bailey Sussman foundation for their financial support, without which my internship and the completion of this research would not be possible. I would also like to thank everyone at the USDA ARS Bio – Integrated Pest Management Unit in Ithaca, New York for their guidance and assistance, particularly my supervisor John Vandenberg. My advisors Melissa Fierke and Christopher Whipps were also invaluable in their help. Novozymes, Inc., Bioworks, Inc., and Barricade International, Inc. graciously provided the commercial products. Additionally, I would like to express my gratitude for the technical assistance of Jonathan Cale, Dylan Parry, Georgia Keene, and the Fierke lab. 7