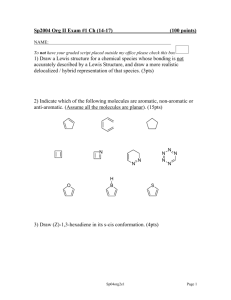
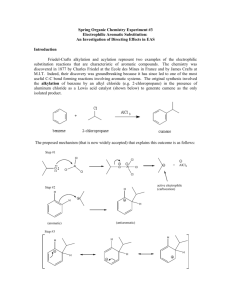
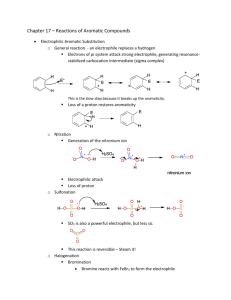
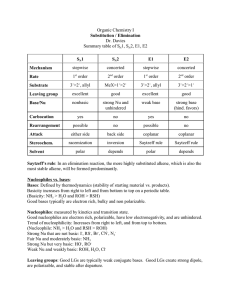
Document 10409950
advertisement
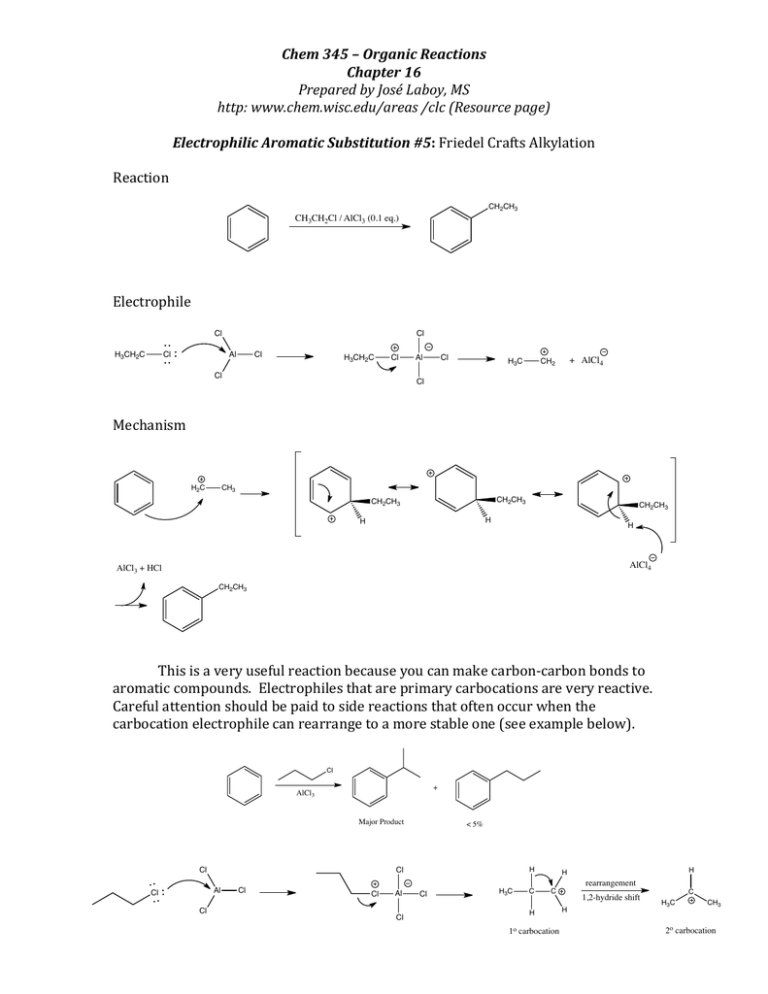
Chem 345 – Organic Reactions Chapter 16 Prepared by José Laboy, MS http: www.chem.wisc.edu/areas /clc (Resource page) Electrophilic Aromatic Substitution #5: Friedel Crafts Alkylation Reaction Electrophile CH2CH3 CH3CH2Cl / AlCl3 (0.1 eq.) Cl H3CH2C Al Cl Mechanism Cl H3CH2C Cl Cl H2C Cl Al Cl H3C + AlCl4 CH2 Cl CH3 CH2CH3 CH2CH3 CH2CH3 H H H AlCl4 AlCl3 + HCl CH2CH3 This is a very useful reaction because you can make carbon-­‐carbon bonds to aromatic compounds. Electrophiles that are primary carbocations are very reactive. Careful attention should be paid to side reactions that often occur when the carbocation electrophile can rearrange to a more stable one (see example below). Cl + AlCl Major Product < 5% H Cl Cl H 3 Al Cl Cl Cl Cl Al Cl Cl H3C C C H 1o carbocation H rearrangement 1,2-hydride shift H C H3C CH3 2o carbocation The Friedel-­‐Crafts reaction only requires catalytic amounts of the Lewis acid because it is recycled through the reaction. There is a major disadvantage of the alkylation reaction and that is over-­‐ alkylation. This is due to the fact that the product of the reaction is activated toward EAS more so than the starting reagent. One way of avoiding over-­‐alkylation is to have an excess of the aromatic reagent and adding the electrophile slowly. Another way of performing alkylation is by using an alkene with an acid catalyst or an alcohol with a Lewis acid catalyst (see below). In each case an electrophile is formed. CH 3 H 2C CH HF OH BF3