Chapter 17 - People.vcu.edu
advertisement

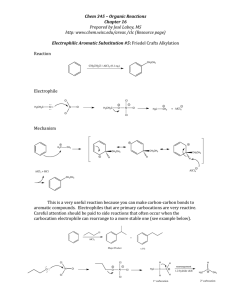
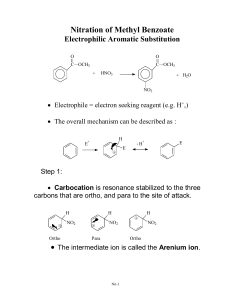
Chapter 17 – Reactions of Aromatic Compounds Electrophilic Aromatic Substitution o General reaction - an electrophile replaces a hydrogen Electrons of pi system attack strong electrophile, generating resonancestabilized carbocation intermediate (sigma complex) E+ E E E This is the slow step because it breaks up the aromaticity. Loss of a proton restores aromaticity E E o Nitration Generation of the nitronium ion H2SO4 nitronium ion Electrophilic attack Loss of proton o Sulfonation H2SO4 SO3 is also a powerful electrophile, but less so. This reaction is reversible – Steam it! o Halogenation Bromination Bromine reacts with FeBr3 to form the electrophile Electrophilic attack Loss of proton Chlorination Just like bromination, but you use chlorine and FeCl3 Iodination Nitric acid is used as a reagent instead of a metal catalyst to generate I+ o Friedel-Crafts Alkylation Alkyl choride + AlCl3 form a carbocation-like structure _ AlCl3 Cl-AlCl3 It is important to note that this is likely still complexed with the Lewis acid catalyst. o You don’t really get the free carbocation, but it acts as though you do. The carbocation acts as the electrophile Another way to alkylate When you start with an alkene and add a non-nucleophilic acid, you can generate a carbocation. This carbocation can now be the electrophile for Alkylation. HF Limitations of Friedel-Crafts Alkylation The carbocation can rearrange, so if you’re trying to add a straight chain, this won’t work. Overalkylation o We will see that alkyl groups are activators for this reaction, so once you put one alkyl group on the addition of a second alkyl group is even easier. Does not work on deactivated rings. o Friedel-Crafts Acylation AlCl3 removes the Cl from an acid chloride _ AlCl3 AlCl3 This cation is resonance-stabilized, so you really do get it. This is called the acyllium ion and you do need to know its name! The carbonyl and alkyl group add to the ring How Friedel-Crafts Acylation gets over two of the limitations of Alkylation No rearrangement You will not overacylate, because once you put the acyl group on, you have deactivated the ring It still does not work on deactivated rings. Name of Reaction Reagents Electrophile What Replaces the H Nitration HNO3/H2SO4 Nitronium ion NO2 (NO2+) Sulfonation H2SO4 SO3 or HSO3+ SO3H (SO3 sometimes) Bromination Br2, FeBr3 “Br+” Br Chlorination Cl2, FeCl3 “Cl+” Cl Iodination I2, HNO3 I+ I Alkylation RCl/AlCl3 or RBr/FeBr3 “R+” R Acylation Acid chloride/ AlCl3 Acyllium ion R R Substituent Effects o We talked about Electron Donating Groups (EDGs) vs. Electron Withdrawing Groups (EWGs) in Chapter 15 when talking about Diels-Alder Which category would make this reaction go faster? The slow step is the step where the electrons of the aromatic system attack the electrophile, so having greater electron density would make this slow step more likely to occur. As such, we will call EDGs “activating” and EWGs “deactivating” o Halogens are weird. We’ll see why. o Ortho, para-directors Ortho, para-directors are EDGs. Why? Think again about that sigma complex. Let’s look at brominating phenol. Not only does having an EDG on the positively charged carbon stabilize by induction, but when the EDG donates by resonance as well, you also get an additional resonance form. A similar series of drawings can be made with para-addition When you halogenate at a meta position, the positive charge is never stabilized by the substituent. The majority of ortho, para-directing groups are activating as well. The exception is halogens. o Meta-directors All meta-directors are deactivating EWGs. If you put an EWG at an ortho or para position, then you destabilize the sigma complex. o Multiple substituents When possible, make everyone happy. o-, p-director Cl2, AlCl3 m-director When you can’t make everyone happy, the most activating group determines the placement of the electrophile. This makes sense because basically, the activating groups want this reaction to happen, and the deactivating groups don’t want it to happen. The further to the right below “wins” the fight over where to put the new piece. strong deactivators weak deactivators weak activators strong activators -NO2 -NR3+ -OH halogens Carbonyls alkyl groups -CN -NH2 most activating least activating -OR Side-chain reactions o Clemmenson Reduction Chops off the carbonyl of ketones Zn(Hg) HCl (aq) Useful when you want to add a straight chain alkyl group. First, acylate, then reduce. o Wolff-Kishner Reduction Overall, it’s the same reaction as Clemmenson reduction, just with different reagents. N2H4 - OH o Benzylic Oxidation If the benzyllic carbon has at least one hydrogen on it, then treatment with KMnO4 or chromic acid chops the rest of the chain off and turns the first carbon into a carboxylic acid. KMnO4 o Halogenation goes faster at benzylic positions. o SN1/SN2 both go faster at benzylic positions. Synthesis questions o When asked to synthesize something from benzene, the task can often seem daunting. o It’s important to remember that the more difficult synthesis questions of this material fall into two categories: Two meta-directors ortho or para to each other COOH ? O2 N Step 1: Alkylate AlCl3 Step 2: Put the other piece on HNO3 H2SO4 Step 3: Oxidize O2 N COOH KMnO4 O2 N O2 N Two ortho-, para-directors meta to each other ? Step 1: Acylate AlCl3 Step 2: Put the other piece on I2 HNO3 Step 3: Reduce Zn(Hg) HCl (aq) For both of the above schemes, remember that there could be some other intervening steps. Nucleophilic Aromatic Substitution o Addition-Elimination Recognizing that you will be doing this reaction There has to be a halogen on the ring. Vigorous, basic conditions Strong EWG’s ortho and/or para to the halogen Only time where fluorine works best! o Because fluorine is the most electronegative, it creates a stronger partial positive at the carbon, so the nucleophilic attack is more likely to happen. Overall Reaction The base replaces your halogen and nothing else happens. Step one: Strong nucleophile adds to the ring, generating carbanion intermediate NO2 NO2 - OH NO2 NO2 OH - NO 2 NO2 Step two: Leaving group leaves, regenerating aromaticity. NO2 NO2 OH OH - NO2 NO2 NO2 NO2 o Elimination-Addition (Benzyne) Overall reaction: Halogen leaves and strong base goes where the halogen was or one away in either direction. Recognizing that this is the reaction you’re doing Halogen on the ring Megabase (most likely NH2-) Most likely something else on the ring acting as a place-holder. Step one: Base and substrate undergo E2-like reaction. -NH 2 benzyne This really happens in two steps, not 1. Step two: Base attacks benzyne intermediate and proton is picked up at other side of triple bond. - NH2 NH2 H+ Halogen’s relationship to other substituent Possible products Ortho Ortho, Meta Meta Ortho, Meta, Para Para Meta, Para A weird reaction of phenols! OH _ COO_ _ OH H+ OH COOH COOH