The Geometric Clutch as a Working Hypothesis for Future Research
advertisement

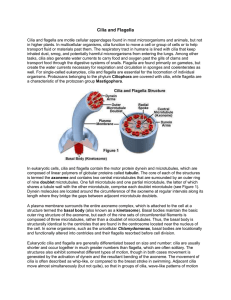
The Geometric Clutch as a Working Hypothesis for Future Research on Cilia and Flagella CHARLES B. LINDEMANN Department of Biological Sciences, Oakland University, Rochester, Michigan, USA ABSTRACT: The Geometric Clutch hypothesis contends that the forces transverse to the flagellar axis (t-forces) act on the axonemal scaffold to regulate flagellar beating. T-forces develop as the product of the curvature and the accumulated tension or compression on the doublet microtubules. In this respect, t-force is a mediator of self-organizing behavior. It arises from the collective action of the assemblage of dynein motors on the structural components of the axoneme and, in turn, imparts order to the sequence of activation and deactivation of the dynein. At the switch point of the flagellar beat, the magnitude of the t-force per micron of flagellum is approximately equal to the sum total of dynein force that can be generated per micron of flagellum. This suggests that the t-force could directly overcome the force-producing dynein bridges and terminate their action. However, many questions remain to be answered concerning the behavior of the axonemal scaffold under stress. Little is known of the force-bearing capacity of the radial spokes and the central pair (cp) projections. The properties of these structures will determine how t-force is distributed within the axoneme. The mechanical and elastic properties of the dynein arms and nexin links need to be better understood to determine how they respond to the application of t-force. In the framework of the Geometric Clutch hypothesis these are the issues that are most important to explore if we are to understand how the flagellum works. KEYWORDS: flagella; cilia; sperm; t-force; dynein; axoneme; radial spokes; nexin links INTRODUCTION Cilia and flagella are motile organelles of eukaryotic cells. They are whiplike appendages of the cell that exhibit rhythmic beating and in doing so exert propulsive force on the surrounding fluid. The mechanism that generates the Address for correspondence: Charles B. Lindemann, Department of Biological Sciences, Oakland University, Rochester, Michigan, USA, 48309. Voice: 248-370-3576; fax: 248-370-4225. lindeman@oakland.edu C 2007 New York Academy of Sciences. Ann. N.Y. Acad. Sci. 1101: 477–493 (2007). doi: 10.1196/annals.1389.024 477 478 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES beat of cilia and flagella has posed an enduring research problem. The curious action of ciliary motion was noted in research reports as early as 1835,1 yet the underlying mechanism that generates the beat is still incompletely understood today. We now know some important things about the underlying motor mechanism of cilia and flagella. The internal framework of a cilium or flagellum consists of a circular arrangement of nine outer doublet microtubules surrounding a central pair (cp) of microtubules.2–4 This scaffold of microtubules, that is common to most cilia and flagella, is called the axoneme. FIGURE 1 illustrates our present conception of the structures in the axoneme as viewed in cross-section. Each of the outer doublets has armlike projections that extend toward the next doublet in the circle; these are called the inner and outer arms. The motive force for the flagellar beat is provided by the molecular motor dynein, first identified by Gibbons and Rowe in 1965, and shown to be the major protein of the armlike projections.6 The base of the flagellum anchors the outer doublets. In most cilia and flagella, this is accomplished by the basal body, although mammalian sperm anchor the doublets in a structure called the connecting piece. In addition, elastic linkages, called the nexin links, resist the free sliding of the doublets. Summers and Gibbons showed that flagella broken off of their base and subjected to brief digestion with trypsin (to cut the nexin links) would slide apart in the presence of Mg-ATP, telescoping to eight times their original length.7 Therefore, the basic motive interaction in the flagellar structure is the sliding of the doublets due to the action of the dynein motor proteins in the presence of Mg-ATP. This sliding is converted to bending by the restraining influence of the basal anchor and the nexin links. FIGURE 1. A schematic diagram of the flagellar axoneme in cross-section. The structures that are discussed in this report are labeled for the convenience of the reader. The details of the cp complex are based on the work of D.R. Mitchell.5 LINDEMANN 479 Sale and Satir showed that the direction of sliding is uniform around the axoneme with the dyneins of each doublet translocating that doublet baseward on the neighboring doublet.8 Therefore, the dyneins on one side of the axoneme tend to bend the flagellum in one direction of the beat cycle and the dyneins on the opposite side contribute to bending in the opposite direction. In most (but not all) flagella and cilia, the doublets numbered 5 and 6 in the standard numbering convention are permanently linked to one another and cannot slide relative to each other2 and the cp is positioned perpendicular to the major plane of the beat.3,9 These structures provide landmarks that define the beat plane, indicated by the double-headed arrow in FIGURE 1. Consequently, the dynein bridges starting with doublet 1, located at approximately 90 degrees to the plane of the cp on one side of the axoneme, is the first of the group 1 through 4 whose dyneins bend the axoneme in one direction of the beat cycle. The opposing group is the dyneins on doublets 6 through 9, which bend the flagellum in the opposite direction. It is this far in the story that most authorities are in agreement. Satir proposed that the beat consists of alternate episodes of activation of the two opposing dynein bridge sets, regulated by some means of switching one set “on” and the other set “off” at appropriate mechanical set points.10–12 This was postulated as the “switch point” hypothesis and is generally believed to be a valid summary of the known data into an orderly picture of the events in the beat. It is at this point in our story that paths diverge and our understanding becomes incomplete. Quite naturally the next thing that needs to be resolved is, what is the control for the switching of the two dynein bridge sets? We know that the control must reside in the axoneme itself. Brokaw demonstrated that the flagellum removed from the intact cell was capable of autonomous functioning.13 Later, it was shown that even isolated pieces of flagellum retain the capacity for propagating a bend and sustaining a rhythmic beat if supplied with Mg-ATP.14,15 One of the most fascinating observations in these early studies was that cut-off pieces of flagella that lost coordination and stopped beating could be restarted by bending the flagellum with a microprobe.14 This observation has been revisited several times by other investigators and appears to be a universal characteristic of the axoneme.16–18 If a hypothesis is to successfully explain how the axoneme works it must be able to explain this inherent mechanical sensitivity of the axoneme. The Geometric Clutch hypothesis19–22 provides a putative mechanism that is compatible with the observed mechanical sensitivity of the axoneme and is also consistent with many experimental observations.23–25 In simplest terms, the hypothesis is based on the following idea: In an intact axoneme, the motor proteins are positioned just far enough from their binding sites on the adjacent doublet that when the flagellum is straight, and at rest, the dynein heads have a very small, but finite, probability of forming a bridge to the next doublet. The probability of forming a bridge increases if the interdoublet distance is decreased. A bend in the flagellum stretches the nexin links 480 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES that are between the doublets and produces a force transverse to the flagellum that squeezes some doublets toward each other. Recently, Morita and Shingyoji showed that bending the axoneme can directly control the activation of sliding between the doublets in a reversible manner,26 supporting this view. The transverse force (t-force) in a passive flagellum can be calculated if the value of the nexin elasticity is specified. When dynein bridges attach in the presence of Mg-ATP, they translocate upon the adjacent doublet and apply a longitudinal force to both of the doublets involved. The longitudinal force exerted across the diameter of the axoneme provides the torque required to actively bend the flagellum. As the flagellum bends, the curved doublets that are under tension (or compression) experience a t-force that acts to pry them apart. When this t-force component becomes sufficiently large, the dynein proteins can no longer continue to function as force-producing motors because they are pulled away from their binding sites on the adjacent doublet. Viewed in the context of the Geometric Clutch hypothesis, the axoneme is a structure that has as its most important function the management and distribution of tension derived from the dynein motors. Consequently, the axoneme must do several interrelated things. Firstly, it must sum up the forces from many dynein motors and apply that accumulated tension across the cross-sectional diameter of the axoneme to create the torque that bends the flagellum and produces movement. Secondly, it must summate and transfer the forces in such a way that the dynein itself is turned on and off periodically. This involves the production of t-force and the application of the t-force to the dynein motors in the right amount to activate and deactivate their motor function. Thirdly, the axoneme construction must support the integrity of the ninefold structure while transferring and applying the summated tension generated by the motors. This report will examine some of the interesting questions that follow from viewing the axoneme from this perspective and suggest some of the key issues that may be resolved by experimentation. THE HYPOTHESIS The Geometric Clutch hypothesis can be summarized as follows: The t-force that develops between the outer doublets and across the axoneme is the primary regulator that controls the activation and deactivation of the dynein motors to produce the beat cycle. This statement subsumes additional details that are required to understand the implied working mechanism. To begin with, the t-force requires some description, both in its origin and its application to the axoneme. The t-force results from two contributing sources, a local component and a global component. The local component of the t-force acting on the doublets comes directly from the transverse vector component of tension (or compression) acting on all linkages to the doublets. Connections to the doublets that are capable of LINDEMANN 481 contributing t-force are the nexin links connecting the doublets, and the radial spoke connections to the cp projections, and the dynein arms when they are bound to the adjacent doublet. When the axoneme is bent, the inter-doublet nexin links are stretched by inter-doublet shear (i.e., sliding) and the stretch generates a tension on the link. The transverse component of that elastic tension acts to pull the doublets together and is therefore a component of the t-force. When the dyneins are active they contribute both a longitudinal and a transverse component of motive force due to the geometry of the dynein linkages (see Ref. 27 for an exposition of this effect). In addition to the local contributions to the t-force, there is a global component that is in some ways more unexpected and more interesting. When active dyneins generate the force that translocates the doublets, the longitudinal force on the doublets is transmitted to the basal anchor (basal body or connecting piece) and results in linear tension on the doublet. This linear tension accumulates as an integral sum of the contributions of participating dyneins along the length of the doublet. Similarly, the stretched nexin links contribute a vector component of tension that is longitudinal to the doublet. This tension also accumulates on the doublet. As a consequence of this process of summation, the longitudinal tension on a doublet can be quite large. When a linear structure, like a microtubule, is curved and under tension (or compression), a transverse component of the tension develops that must be restrained by supporting structures. The magnitude of this t-force component is equal to the longitudinal force multiplied by the curvature.19 Calculations show that in a sea urchin flagellum the global component of the t-force that will develop in a typical flagellar bend is quite large, on the order of 0.5 nN/m.27 This is approximately 100 times greater than the local t-force from the nexin link stretch in the same bend. The magnitude of the global component of the t-force is such that, in both sea urchin and bull sperm flagella, the t-force acting on 1 m of flagellar length at the switch point of the beat is roughly equal to the combined force that all the dyneins in that same 1 m of length are capable of generating.27 Therefore, it is not hard to imagine that the t-force could be overwhelming the dyneins at the switch point of the beat. This raises the first issue that is amenable to direct scientific investigation: 1. How do dynein motors react to applied force? If the Geometric Clutch hypothesis is used as a plan to guide experimental investigation, the first and most basic issue that should be investigated is the relationship of t-force to dynein function. It would be very informative to know how much force it takes to terminate dynein cross-bridge attachment to tubulin. When the dyneins are located on a doublet, how much t-force does it take to terminate the processive sliding of that doublet on a second doublet? The current sophistication of optical trap and atomic force microscope (AFM) techniques may allow this question to be addressed directly. The issue of the dissociation force for dynein has another dimension. The Geometric Clutch computer model19,20 produces oscillations by assuming there 482 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES is hysteresis in the behavior of a group of dynein molecules. Uniformly crossbridged dyneins, as visualized in FIGURE 2A, will remain attached and resist the t-force to a fairly high threshold. This allows a substantial bend to develop before switching ensues. If this were not so, the beat cycle would not produce sufficient bending to be effective for propulsive motility. However, once the dyneins do begin to disengage, as shown in FIGURE 2B, the disengagement must proceed until a much lower t-force value is reached, or the dyneins will not deactivate sufficiently to allow the beat to reverse direction. Hysteresis in the “on” and “off” set points is a necessary property of all relaxation oscillators. According to the Geometric Clutch hypothesis, the oscillation we call the flagellar beat cycle is produced by hysteresis in the t-force set point for activating and deactivating the dynein motors. This is a property of dynein that needs to be verified experimentally. What this will require is to show that the force necessary to disengage the dyneins drops dramatically when a gap develops in a length of active dynein FIGURE 2. The interaction of t-force with dynein. (A) When two adjacent doublets are uniformly bridged by dynein cross-bridges, applied t-force (arrows) will be distributed over many dynein linkages and resistance to dissociation should be highest. (B) At a boundary where dyneins are making the transition between the bound (bridged) state and the free (released) state, the effect of t-force will be concentrated at the dynein units at the transition (dashed lines). (C) Discontinuity in the uniformity of attached dynein bridges will have the effect of concentrating the action of the t-force on the dyneins in the transition zones (dashed lines). This behavior will reduce the t-force needed for dynein disengagement whenever a bridging discontinuity develops. This provides the hysteresis that is required for the beat oscillation in the Geometric Clutch mechanism. LINDEMANN 483 motors, as illustrated in FIGURE 2C. The kind of experiment undertaken by Kamiya and Okagaki with frayed axonemes offers the opportunity to observe the kinetics of dyneins on an intact doublet pair.28 Recently, Aoyama and Kamiya revisited that experimental protocol with a new focus on the Geometric Clutch hypothesis.29 They found that there are at least two, and possibly three, dissociation patterns that are observed when the doublets separate. This could be an indication that the dissociation threshold is conditional on the number and pattern of functional dyneins that remain on the doublets. It also suggests that the t-force required to separate two doublets might be conditional on the pattern of dynein attachment. Building on this method might be one way to answer the question of hysteresis in the dynein response to t-force. Another possibility would be to use optically trapped beads to pull apart a microtubule that is translocating on the dyneins of an isolated outer doublet. We also do not know how the dynein bridge between adjacent doublets responds to the application of t-force. Goodenough and Heuser produced some striking micrographs showing the outer dynein arms in rigor and in the relaxed condition. 30,31 Additionally, Burgess showed that in images of cryofixed motile flagella the rigor and relaxed configurations appear to alternate.32 In the rigor configuration the globular dynein head appears to nest in two protein densities that they called the P-foot and the D-foot, as shown in FIGURE 3A. When the axoneme was subjected to stress during preparation, Goodenough and Heuser showed that the dynein head would undock from its position between the feet and would end up suspended in the inter-doublet space.31 It is likely that this is similar to the action that t-force exerts on the native dynein bridges, as shown schematically in FIGURE 3B. Based on an analysis of the stiffness of the stem of the dynein molecule, Lindemann and Hunt proposed that the dynein heavy chain could not support force transmission without additional support.33 The nesting of the head in the P- and D-feet as seen in the micrographs of Goodenough and Heuser would provide just the kind of support needed for force transmission during the power stroke.30 Elimination of this support by pulling the head away from its docking position could be the mechanism whereby the t-force deactivates the dynein motors. Applying the same quick-freeze deep-etch technique to rapidly frozen beating flagella might confirm that such a transition to the undocked state is present in the strongly bent regions of actively beating flagella. If, in fact, the axoneme is using t-force to switch the dyneins, then the t-force will be acting to distort the axoneme during the beat cycle. When the dyneins release from their cross-bridged active configuration the doublets should recoil away from each other in response to the unsupported t-force acting on the nexin links and spokes as illustrated in FIGURE 4. This raises the second issue for experimental verification: 2. Does the axoneme distort during the beat cycle? Recently, Sakakibara et al. used AFM to demonstrate that the action of the dynein motors results in an oscillation of the axoneme diameter in immobilized 484 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES FIGURE 3. A possible mechanism for the interaction of the t-force with the dynein power stroke. Alternation of the rigor and relaxed configuration of the dynein cross-bridge as illustrated in (A) is the condition seen in electron micrographs of the active axoneme as reported by Burgess.32 As suggested by Lindemann and Hunt, 33 a stabilized condition of the dynein head, as occurs in the rigor configuration, may be a requirement for the transmission of force between the doublets during the power stroke (PS). When the bridge is in the relaxed configuration, ATP is bound and the B-link is in the low affinity state where relocation (RL) to a new binding site on the B-subtubule takes place. Alternation of the rigor and relaxed states leads to inter-doublet sliding. Application of t-force, as shown in (B), increases the inter-doublet spacing31 and prevents the formation of the rigor complex, thereby preventing the step in the bridge cycle that is required for inter-doublet force transmission. sea urchin flagella.34 This would indicate that engagement of the dynein motors alters the inter-doublet spacing, which is consistent with the Geometric Clutch hypothesis. Additionally, the transmission electron microscope (TEM) images presented by Mitchell using a fixation technique that successfully preserved the waveform of the Chlamydomonas beat, appear to show that the axoneme becomes wider in the bent regions.35 This is exactly what would be expected if the t-force were distorting the axoneme, as in FIGURE 4. While these observations are suggestive, more definitive evidence is needed to confirm that the inter-doublet distances change during the normal beat cycle. The changes in spatial relationships within the axoneme of active flagellar or cilia should be visible in freeze fracture replicas of fast frozen flagella or cilia, such as those so elegantly presented by Goodenough and Heuser.30,36 The new technique of Cryo-EM tomography should also be capable of seeing the change in doublet separations if vitrified specimens of active flagella are examined. Application of this technique to quiescent flagella has already been extraordinarily valuable.37 LINDEMANN 485 FIGURE 4. Distribution of t-force and axonemal distortion. When the dyneins on one side of the axoneme engage and generate tension to bend the flagellum, as shown in (A), most or all of the tension ends up accumulated on doublet 1 and doublets 5 and 6. As a consequence most, or all, of the global t-force (see text for description of global t-force) will be exerted across the axoneme from doublet 1 to doublets 5 and 6. This positions the t-force to put stress on the cp and spoke linkages as well as the attached dyneins. Which of these two pathways experiences the greater share of the t-force tension will depend in great measure on the mechanical properties of the spoke–cp axis. After the dynein bridges release, as shown in (B), all of the t-force must be held by the spoke–cp axis and the nexin links. This will produce a distortion of the axoneme as illustrated in (B). The extent of the distortion will depend on the mechanical properties of the spoke–cp axis. Adapted from Lindemann27 ; reproduced with permission. Another major issue concerns the way that the axoneme structure interacts with applied tension to create and distribute the t-force. The summation of tension on the doublets is only possible if the doublets are relatively inextensible and anchored. Brokaw’s observations on detergent extracted flagella tagged with beads seem to support the concept that the doublets are fairly inextensible under normal loading.38,39 Therefore, the doublets should be capable of transmitting most of the dynein-generated tension to the basal anchor. The next issue is: 3. Does the basal anchor alter the beat cycle? Without a basal anchor a doublet would experience considerably less accumulated tension, and hence, would develop considerably less t-force. In addition, the distribution of t-force in the absence of a basal anchor would not favor switching to begin near the basal region of the flagellum, but closer to the middle of the axoneme or, possibly, not at all. This relationship is illustrated in FIGURE 5. The reduction in t-force in the absence of a basal anchor would decrease the likelihood of a beat cycle; consequently, spontaneous beating might not develop, especially in cilia and flagella of short or moderate length. The effect of removing the basal body has been widely observed, as reviewed by Goldstein.40 When oscillation is still achieved, the basal to tip direction of wave propagation should be altered or compromised. The recent studies of 486 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Fujimura and Okuno seem to support the Geometric Clutch interpretation of the role of the basal anchor and show that beating and bend propagation can be restored without a basal body as long as the doublets are anchored.41 The Geometric Clutch mechanism suggests that anchoring the doublets at the tip rather than the base should also be able to restore beating, and in that case the direction of bend propagation should be reversed, as illustrated in FIGURE 5. This prediction remains to be tested. When a flagellum or a cilium beats, the dyneins on the doublets of one hemicircle of the axoneme must work together to generate the tension that bends the flagellum. This means that in most cilia or flagella the dyneins on doublets 1, 2, 3, and 4 work together to bend the axoneme in one direction and the dyneins on doublets 6, 7, 8, and 9 work together to bend the axoneme in the FIGURE 5. The role of the basal anchor. (A) In an intact flagellum, an episode of dynein engagement bends the flagellum by accumulating the tension at the basal anchor (i.e., the basal body/connecting piece). The resulting t-force is largest near the basal end of the flagellum and begins the process of dynein disengagement that leads to bend propagation in a direction away from the basal anchor. (B) In the absence of a basal anchor tension can only be accumulated on the doublets by the resistance of a large number of resisting nexin links. Therefore, a bend can only develop if the dyneins at one end of the axoneme are acting against the combined resistance of the nexin over an extended distance. Under these circumstances bending will be more centrally located and much more gradual. This results in a much reduced t-force and a reduced chance of a successful beat cycle developing. (C) Restoration of a mechanical anchor, even if it is at the wrong end, should increase the probability of a beat cycle developing. If the Geometric Clutch mechanism is correct, the direction of bend propagation should be reversed by introduction of a distal anchor. LINDEMANN 487 opposite direction (see FIG. 4). The force that is contributed by the concerted action of each of these two dynein groups ends up as tension and compression on doublets 1 and 5–6. In the flagella and cilia of metazoans, the 5–6 doublets are permanently bridged and act as a unit. Consequently, all (or most) of the tension contributed by the dyneins ends up directed across the center of the axoneme. This also means that most, or all, of the t-force that develops acts across the central axis as well. Since the radial spokes from doublets 1 and 5–6 appear to interact with the cp projections this raises the question of the role of the spoke–cp axis in the distribution of the t-force, as illustrated in FIGURE 4. There are many unanswered questions regarding the role of the spoke–cp axis in managing the t-force. In the context of the Geometric Clutch mechanism this is a crucial issue; the points of major interest are illustrated in FIGURE 6. The flexibility of the cp projections could play a role in governing the distribution of t-force between the dyneins and the spokes. The more the t-force that is carried by the spoke–cp axis, the less t-force the dynein motors will experience; thus, a greater curvature will develop before dynein disengagement occurs. This puts the spokes and cp apparatus in an ideal regulatory position. Release of the spoke head attachments to the cp projections would cause dramatic redistribution of t-force from the spokes to the dyneins. This may be an important step in the dynein-switching mechanism. Therefore, the most fundamental issue to be addressed is: 4. Do the spokes form physical attachments to the cp projections, and if they do, how much force can that attachment hold? This is a most fundamental interaction and is a crucial determinant of how the axoneme distributes t-force. We know from the work of Warner and Satir that the spokes readily tilt and the spoke heads translocate to new positions on the cp apparatus as the axoneme bends.42 Remarkably, we know almost nothing else about the spoke head–cp interaction. FIGURE 6 illustrates the components of the spoke–cp axis and properties of that axis that need to be discovered. In a typical bending wave of a sea urchin sperm, the spokes of doublet 1 must experience a t-force of approximately 7pN/ spoke immediately after switching.27 Before the dyneins bridges release, the t-force experienced by the spokes on doublet 1 may even be higher, but will depend on how much the t-force is resisted by the attached dynein bridges. How does the spoke head attachment to the cp projections handle the t-force? Does the spoke head remain attached to the cp projections or does the spoke head pull away from the cp projections under loading? Yang et al. succeeded in isolating intact spokes,43 and Kamiya devised a method to isolate cp complexes.44 Do isolated spokes have the ability to bind the isolated cp complexes? This is a most important missing piece of information. Furthermore, if the spoke heads do bind, it should be possible to determine the force required to separate them using a dual beam laser trap methodology. Real cilia and flagella can change their beating pattern in response to Ca2+ , an effect that is mediated by a direct action of calcium ion on the axoneme.45,46 488 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES FIGURE 6. Mechanics of the spoke–cp axis. Most of the t-force that develops in an intact flagellum during the beat cycle will be directed across the spoke–cp axis. The components of that force-bearing axis are shown here in isolation. We know almost nothing of the mechanics of this structure that is so strategically positioned to interact with the t-force. Questions that need to be addressed are: (A) Is the spoke shaft extensible and how are its properties changed by the four calmodulin units located on the shaft? (B) Does the spoke head form a bond to the cp projections and, if it does, how mechanically strong is the linkage? (C) Are the cp projections flexible and elastic? How much axonemal distortion is possible without detaching the spoke head from the cp projections? All of these factors will impact the distribution of t-force between the spoke–cp axis and the dynein, and may be the key to understanding the control of the amplitude of the beat. At high concentrations of free calcium ion, detergent-extracted and reactivated sea urchin sperm will arrest in a strongly curved configuration.47 The axoneme is lavishly decorated with proteins that are calcium response elements. Calmodulin has been localized to the spokes43,48,49 and there is some evidence that the spokes can inhibit microtubule sliding in the presence of Ca2+ .50 Centrin, a Ca2+ activated contractile protein, has been localized to the dynein regulatory complex (DRC).51–53 How is it that calcium ion is able to change the switching parameters of the beat to modify the waveform? The Geometric Clutch mechanism offers several concrete possibilities that could be tested: LINDEMANN 489 5. Does Ca2+ change the length or the tensile strength of the spoke? 6. Does Ca2+ change the binding of the spoke head to the cp complex? The fraction of the t-force that can be transmitted through the spokes and cp complex directly alters the t-force that the dynein motors experience. Therefore, any change in the spoke length or change in the elasticity or tensile strength of the spoke–cp axis will alter the switch point of the beat. Changing the switch point through such a spoke-dependent mechanism would change the amplitude and wave propagation characteristics of the flagellum. In other words, the waveform would be altered. The Geometric Clutch computer model informs us that a change in the probability of the dynein motors finding and attaching to a binding site on the B subtubule of the adjacent doublet can have powerful effects on the waveform of the resulting beat.20,22,24 Centrin (also called caltractin) is a calcium response element that changes its molecular conformation when calcium is bound. It is located at the attachment point of two inner row dynein heavy chains.53 In this location it could change the projection angle of an inner row dynein and thereby alter the probability of the dynein forming a spontaneous bond to the B subtubule. The centrin calcium response elements are in the DRC, FIGURE 7. The projection angle of the inner arm dyneins. The Geometric Clutch model predicts that small changes in the probability of spontaneously forming a dynein cross-bridge attachment will make a very significant impact on the waveform of the beat.54 Cross-bridge attachment probability would be sensitive to minor adjustments of the angle of dynein projection from the A subtubule, as illustrated. The inner row of dynein arms, which have the greatest impact on the waveform of the beat, is anchored to the A subtubule at, or near, the DRC as illustrated in (A). A structural modification of a protein in that complex, for instance in response to Ca2+ binding as illustrated in (B), could alter the projection angle of one or more of the inner arm dyneins and produce the necessary change in bridge attachment probability required to explain phenomena, such as calcium regulation of the beat symmetry. 490 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES and associate with dynein arms I2 and I3.53 This raises another issue that is amenable to investigation: 7. Is there a calcium dependent conformational change in the projection angle of the dynein arms from the A subtubule? This would be especially relevant to the question of waveform symmetry if there are asymmetries in the conformation of the dyneins around the ring of nine doublets. If the projection angle of some of the dynein heavy chains on one side of the axoneme was altered by calcium ion, as illustrated in FIGURE 7, this would be the kind of conformational change that the Geometric Clutch mechanism requires to make the symmetry of the beat vary with the calcium concentration. There is already some evidence that there is a radial asymmetry in the inner dynein arm region of the Chlamydomonas axoneme,55 but definitive structural data are wanting. CONCLUSIONS The Geometric Clutch hypothesis instructs us to view the axoneme as a machine for carrying tension and managing t-force. In this regard, the hypothesis provides a framework that can be used to make predictions about the functioning of the axoneme components and to suggest useful experiments. Seven items that are amenable to experimental investigation are identified in this report. Exploration of these specific questions would facilitate a greater understanding of the working mechanism of cilia and flagella. ACKNOWLEDGMENTS The author wishes to thank Ms. Kathleen A. Lesich for assistance in the preparation of the figures and manuscript. This work was supported by grant MCB-0516181 from the National Science Foundation. REFERENCES 1. SLEIGH, M.A. 1962. The Biology of Cilia and Flagella. Pergamon. Oxford, UK. 2. AFZELIUS, B.A. 1959. Electron microscopy of the sperm tail. Results obtained with a new fixative. J. Biophys. Biochem. Cytol. 5: 269–278. 3. AFZELIUS, B.A. 1961. The fine structure of the cilia from ctenophore swimmingplates. J. Biophys. Biochem. Cytol. 9: 383–394. 4. GIBBONS, I.R. & A.V. GRIMSTONE. 1960. On flagellar structure in certain flagellates. J. Biophys. Biochem. Cytol. 7: 697–716. 5. MITCHELL, D.R. 2003. Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Motil. Cytoskeleton 55: 188–199. LINDEMANN 491 6. GIBBONS, I.R. & A.J. ROWE. 1965. Dynein: a protein with adenosine triphosphatase activity from cilia. Science 149: 424–426. 7. SUMMERS, K. & I.R. GIBBONS. 1971. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc. Natl. Acad. Sci. USA 68: 3092–3096. 8. SALE, W.S. & P. SATIR. 1977. Direction of active sliding of microtubules in Tetrahymena cilia. Proc. Natl. Acad. Sci. USA 74: 2045–2049. 9. GIBBONS, I.R. 1961. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J. Biophys. Biochem. Cytol. 11: 179–205. 10. SATIR, P. 1985. Switching mechanisms in the control of ciliary motility. Mod. Cell Biol. 4: 1–46. 11. SATIR, P. 1989. Regulation of ciliary and flagellar movement. In Cell Movement. The Dynein ATPases. F.D. Warner, P. Satir & I.R. Gibbons, Eds.: 253–256. Alan R. Liss. New York. 12. SATIR, P. & T. MATSUOKA. 1989. Splitting the ciliary axoneme: implications for a “switch-point” model of dynein arm activity in ciliary motion. Cell Motil. Cytoskeleton 14: 345–358. 13. BROKAW, C.J. 1961. Movement and nucleoside polyphosphatase activity of isolated flagella from Polytoma uvella. Exp. Cell Res. 22: 151–162. 14. LINDEMANN, C.B. & R. RIKMENSPOEL. 1972. Sperm flagella: autonomous oscillations of the contractile system. Science 175: 337–338. 15. LINDEMANN, C.B. & R. RIKMENSPOEL. 1972. Sperm flagellar motion maintained by ADP. Exp. Cell Res. 73: 255–259. 16. OKUNO, M. & Y. HIRAMOTO. 1976. Mechanical stimulation of starfish sperm flagella. J. Exp. Biol. 65: 401–413. 17. OMOTO, C.K. et al. 1996. Ability of paralyzed flagella mutants of Chlamydomonas to move. Cell Motil. Cytoskeleton 33: 88–94. 18. HAYASHIBE, K., C. SHINGYOJI & R. KAMIYA. 1997. Induction of temporary beating in paralyzed flagella of Chlamydomonas mutants by application of external force. Cell Motil. Cytoskeleton 37: 232–239. 19. LINDEMANN, C.B. 1994. A “Geometric Clutch” hypothesis to explain oscillations of the axoneme of cilia and flagella. J. Theor. Biol. 168: 175–189. 20. LINDEMANN, C.B. 1994. A model of flagellar and ciliary functioning which uses the forces transverse to the axoneme as the regulator of dynein activation. Cell Motil. Cytoskeleton 29: 141–154. 21. LINDEMANN, C.B. 1996. Functional significance of the outer dense fibers of mammalian sperm examined by computer simulation with the geometric clutch model. Cell Motil. Cytoskeleton 34: 258–270. 22. LINDEMANN, C.B. 2002. Geometric Clutch model version 3: the role of the inner and outer arm dyneins in the ciliary beat. Cell Motil. Cytoskeleton 52: 242– 254. 23. HOLCOMB-WYGLE, D.L., K.A. SCHMITZ & C.B. LINDEMANN. 1999. Flagellar arrest behavior predicted by the Geometric Clutch model is confirmed experimentally by micromanipulation experiments on reactivated bull sperm. Cell Motil. Cytoskeleton 44: 177–189. 24. LINDEMANN, C.B. 2001. Computed simulations of ciliary and flagellar motility using the Geometric Clutch model can replicate a wide variety of experimental conditions. In Computational Modeling in Biological Fluid Dynamics. L.J. Fauci & S. Gueron, Eds.: Vol. 124: 145–165. Springer-Verlag. New York. 492 ANNALS OF THE NEW YORK ACADEMY OF SCIENCES 25. LINDEMANN, C.B. 2004. Testing the Geometric Clutch hypothesis. Biol. Cell 96: 681–690. 26. MORITA, Y. & C. SHINGYOJI. 2004. Effects of imposed bending on microtubule sliding in sperm flagella. Current Biol. 14: 2113–2118. 27. LINDEMANN, C.B. 2003. Structural-functional relationships of the dynein, spokes, and central-pair projections predicted from an analysis of the forces acting within the axoneme. Biophys. J. 84: 4115–4126. 28. KAMIYA, R. & T. OKAGAKI. 1986. Cyclical bending of two outer-doublet microtubules in frayed axonemes of Chlamydomonas. Cell Motil. Cytoskeleton 6: 580–585. 29. AOYAMA, S. & R. KAMIYA. 2005. Cyclical interactions between two outer doublet microtubules in split flagellar axonemes. Biophys. J. 89: 3261–3268. 30. GOODENOUGH, U.W. & J.E. HEUSER. 1982. Substructure of the outer dynein arm. J. Cell Biol. 95: 798–815. 31. GOODENOUGH, U. & J. HEUSER. 1984. Structural comparison of purified dynein proteins with in situ dynein arms. J. Mol. Biol. 180: 1083–1118. 32. BURGESS, S.A. 1995. Rigor and relaxed outer dynein arms in replicas of cryofixed motile flagella. J. Mol. Biol. 250: 52–63. 33. LINDEMANN, C.B. & A.J. HUNT. 2003. Does axonemal dynein push, pull or oscillate? Cell Motil. Cytoskeleton 56: 237–244. 34. SAKAKIBARA, H.M. et al. 2004. Diameter oscillation of axonemes in sea-urchin sperm flagella. Biophys. J. 86: 346–352. 35. MITCHELL, D.R. 2003. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil. Cytoskeleton 56: 120–129. 36. GOODENOUGH, U.W. & J.E. HEUSER. 1985. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J. Cell Biol. 100: 2008–2018. 37. NICASTRO, D., J.R. MCINTOSH & W. BAUMEISTER. 2005. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proc. Natl. Acad. Sci. USA 102: 15889–15894. 38. BROKAW, C.J. 1989. Direct measurements of sliding between outer doublet microtubules in swimming sperm flagella. Science 243: 1593–1596. 39. BROKAW, C.J. 1990. Flagellar oscillation: New vibes from beads. J. Cell Sci. 95: 527–530. 40. GOLDSTEIN, S.F. 1981. Motility of basal fragments of sea urchin sperm flagella. J. Cell Sci. 50: 65–77. 41. FUJIMURA, M. & M. OKUNO. 2006. Requirement of the fixed end for spontaneous beating in flagella. J. Exp. Biol. 209: 1336–1343. 42. WARNER, F.D. & P. SATIR. 1974. The structural basis of ciliary bend formation: radial spoke positional changes accompanying microtubule sliding. J. Cell Biol. 63: 35–63. 43. YANG, P., D.R. DIENER & W.S. SALE. 2001. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J. Cell Biol. 153: 1315–1326. 44. KAMIYA, R. 1982. Extrusion and rotation of the central-pair microtubules in detergent–treated Chlamydomonas flagella. Prog. Clin. Biol. Res. 80: 169– 173. 45. WALTER, M.F. & P. SATIR. 1978. Calcium control of ciliary arrest in mussel gill cells. J. Cell Biol. 79: 110–120. 46. BROKAW, C.J. 1979. Calcium-induced asymmetrical beating of tritondemembranated sea urchin sperm flagella. J. Cell Biol. 82: 401–411. LINDEMANN 493 47. GIBBONS, B.H. & I.R. GIBBONS. 1980. Calcium-induced quiescence in reactivated sea urchin sperm. J. Cell Biol. 84: 13–27. 48. YANG, C., S. BHATTI & P. YANG. 2002. Initiation codon mutation of RSP2 in Chlamydomonas pf24 mutant leads to reduced expression and defective assembly of radial spokes. Mol. Biol. Cell 13: 327a. 49. SMITH, E.F. & P. YANG. 2004. The radial spokes and central apparatus: mechanochemical transducers that regulate flagellar motility. Cell Motil. Cytoskeleton 57: 8–17. 50. NAKANO, I. et al. 2003. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J. Cell Sci. 116: 1627–1636. 51. PIPERNO, G. et al. 1990. Three distinct inner dynein arms in Chlamydomonas flagella: molecular composition and location in the axoneme. J. Cell Biol. 110: 379–389. 52. PIPERNO, G., K. MEAD & W. SHESTAK. 1992. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 118: 1455–1463. 53. LEDIZET, M. & G. PIPERNO. 1995. The light chain p28 associates with a subset of inner arm heavy chains in Chlamydomonas axonemes. Mol. Biol. Cell 6: 697–711. 54. LINDEMANN, C.B. & K.S. KANOUS 1997. A model for flagellar motility. Int. Rev. Cytol. 173: 1–72. 55. KING, S.J. et al. 1994. The bop2-1 mutation reveals a radial asymmetry in the inner dynein arm region of Chlamydomonas reinhardtii. J. Cell Biol. 126: 1255–1266.