Distribution of Molecular Speeds
advertisement
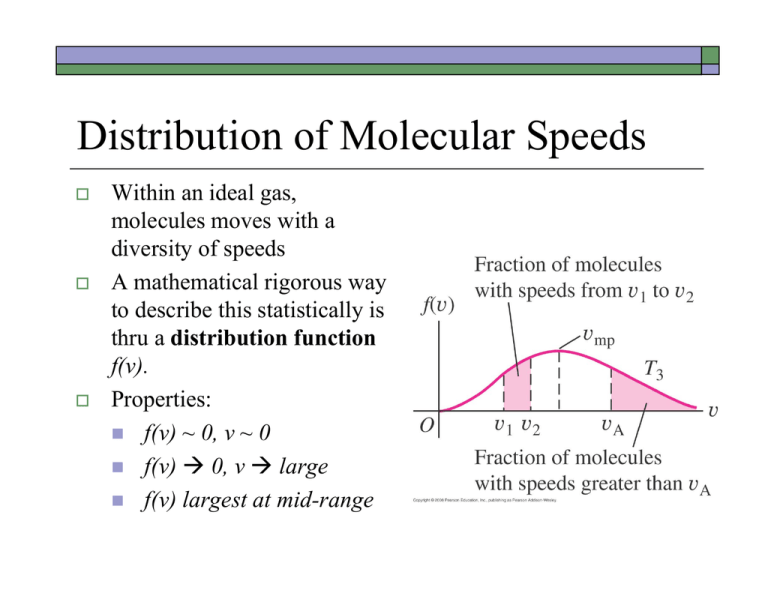
Distribution of Molecular Speeds Within an ideal gas, molecules moves with a diversity of speeds A mathematical rigorous way to describe this statistically is thru a distribution function f(v). Properties: f(v) ~ 0, v ~ 0 f(v) 0, v large f(v) largest at mid-range Maxwell-Boltzmann Distribution m f 4 2 kT 3/ 2 2 m 2 / 2 kT e f(v)dv gives the probability of finding molecules with speed in range [v,v+dv]. Diff averages with respect to the distribution of molecular speeds can be calculated using f(v): 1. vav vf (v)dv (avg of v) 0 2. (v2 ) av 2 f (v)dv (avg of v2) v 0 Notes: Weighted Average 7 Test Scores: si 65,80,80,95,95 , i 1, ,5 Simple Avg: 1 av N s N si i 1 1 65 80 80 95 95 5 sav 15 65 52 85 52 95 sav f (s)s vav f (v)vdv 0 (Weighted Avg) where n(s) = fraction of occurrence of s Notes: Weighted Average 7 Test Scores: si 65,80,80,95,95 , i 1, ,5 Simple Avg: 1 av N s N si2 i 1 1 652 802 802 952 952 5 sav 15 65 52 85 52 95 2 sav f (s)s (v2 ) av 0 2 2 2 (Weighted Avg) where n(s) = fraction of occurrence of s f (v)v2dv Statistical Description of Molecular Speed Average speed (mean value): Root Mean Square (RMS) speed: vav 8kT m kT vrms v2 3m av Note: vav does not equal vrms ! In addition to simple “mean” and “median”, there are other ways to statistically describe the “average” values for a distribution of molecules moving at different speeds! Most Probable Speed - the maximum value of the distribution function f(v): 2kT vmp m Ddrinking Mean Free Path for Gas Molecules r r v 2r Effective collision area = 2 2r (2r ) 2 Assumptions: • Molecule with finite radius r (note: point-like particles do not collide) • The number density (# per unit vol) is given by N/V. • Molecules move at an average speed v (assuming this average to be vrms). For simplicity, let assume that the redder shaded molecule is the only one which is moving and to the right with speed v. Then within a time interval dt, the number of molecules that it might collide will be given by: effective volume dn number density indicated (blue) 4r 2 vdt N V (we take v vrms ) Thus, the number of collisions/unit time is, dn 4 r 2 vN dt V Mean Free Path for Gas Molecules To take into account that other molecules besides the red one are also moving, the estimated collision rate should be higher and it can be shown that this mean collision rate will be larger by 2 . So, dn 4 2r 2 vN dt V note The average time between collisions (mean free time) is then the reciprocal of this value, tmean V 4 2r 2 vN And, the mean free path will be, vtmean V 4 2r 2 N or (we used PV NkT ) kT 4 2r 2 P Derivation of the 2 Factor Key Idea: If all the molecules are moving, the relevant quantity for consideration should be the relative velocity between a pair of molecules. v1 v 2 v1 v2 v 2 v1 Again, we take the average of this relative velocity to be the root-mean-square value: v 2 rel av v 2 v1 v 2 v1 av v 2 v 2 2 v 2 v1 v1 v1 av v 2 v 2 av 2 v 2 v1 av v1 v1 av Note: v1 and v 2 are in random relative directions ! v 2 v1 av 0 Derivation of the 2 Factor 2 This gives, vrel av v 2 v 2 av 2 v 2 v1 av v1 v1 av v22 v12 2 v 2 av av av Again, random direction assumption implies these two averages to be the same ! Taking the square root of the above equation, vrel rms Thus, v 2 rel av 2 v 2 2vrms av dn 4 r 2 2vN dn 4 r 2 vN should be written as dt V dt V back vrms and Example: 18.6 & 18.8 18.6: a) What is the average translational KE of an ideal gas molecule at 27oC? Kinetic Theory KE per molecule 3 3 kT 1.38 1023 J / K 273.15 27 K 6.211021 J 2 2 b) What is the root-mean-square speed vrms of O2 at this T? vrms 3kT mO2 mO2 5.311026 kg (molecular mass) 3(1.38 1023 J / K )(300 K ) 484 m s 26 5.3110 kg 18.8: a) Evaluate the mean free path of an air molecule at 27oC. 1.38 1023 J / K 300 K kT 8 5.8 10 m 2 2 10 5 4 2r p 4 2 2.0 10 m 1.01 10 Pa r 2 10 10 m Dperfume Heat Capacities of Gases (at constant V) Using the Kinetic-Molecular model, one can calculate heat capacity for an Ideal Gas! For point-like molecules (monoatomic gases), molecular energy consists only of translational kinetic energy Ktr We just learned that Ktr is directly proportional to T. When an infinitesimal amount of heat dQ enters the gas, dT increases, and dKtr increases accordingly, dKtr 3 NkdT (or 3 nRdT ) 2 2 Heat Capacities of Gases From definition of molar heat capacity, we also know: dQ nCvdT From energy conservation, requiring dQ=dKtr (no work) gives, nCvdT 3 nRdT 2 Monoatomic ~ Ideal Gas (matches well with prediction) Cv 3 R (12.47 J / mol K ) 2 Heat Capacity (diatomic) A diatomic molecule can absorb energy in its translational motion, its rotational motion and in its vibrational motions. Equipartition of Energy This principle states that each degree of freedom (“separate mechanisms in storing energy”) will contribute (½ kT) to the total average energy per molecule. Monoatomic: 3 translational dofs 3 (½ kT) This give Etot= 3/2 NkT (same as before). Diatomic (without vibration): 3 trans dofs + 2 rotational dofs This give Etot= 5/2 NkT or = 5/2 nRT. Again, consider an infinitesimal energy change, we have nCvdT 5 nRdT, and this gives Cv = 5/2 R. 2 Heat Capacities (real gases, e.g., H2) At low T, only 3 translational dofs can be activated At higher T, additional rotational dofs can be activated At higher T still, vibrational dofs might also get activated Heat capacity for a H2 gas For normal T range, one take Cv 5 R for H2 gas 2 Heat Capacities of Ideal Solids Atoms are connected together by springs Assume harmonic motions for these springs (Hooke’s law) For each spatial direction, there are two dofs (vibrational KE, vibrational PE) and we have 3 dims For the entire solid, we have 3/2 kT (KE) + 3/2 kT (PE) Etot = 3kT Cv = 3R Dulong Petit Prediction and Improvements Einstein: treating atoms as QM HMOs… Cv 0 Debye: extension by including low-f phonons… Cv T 3






