Chapter 13: Temperature and Ideal Gas
advertisement

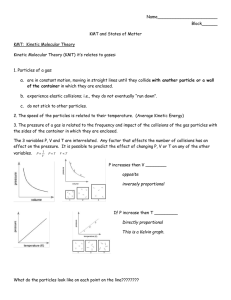
Chapter 13: Temperature and Ideal Gas •What is Temperature? •Temperature Scales •Thermal Expansion •Molecular Picture of a Gas •The Ideal Gas Law •Kinetic Theory of Ideal Gases •Chemical Reaction Rates •Collisions Between Molecules 1 §13.1 Temperature Heat is the flow of energy due to a temperature difference. Heat always flows from objects at high temperature to objects at low temperature. When two objects have the same temperature, they are in thermal equilibrium. 2 The Zeroth Law of Thermodynamics: If two objects are each in thermal equilibrium with a third object, then the two objects are in thermal equilibrium with each other. 3 §13.2 Temperature Scales Absolute or Kelvin scale Fahrenheit scale Celsius scale Water boils* 373.15 K 212 °F 100 °C Water freezes* 273.15 K 32 °F 0 °C Absolute zero 0K -459.67 °F -273.15°C (*) Values given at 1 atmosphere of pressure. 4 The temperature scales are related by: Fahrenheit/ Celsius TF = (1.8 °F/°C )TC + 32°F Absolute/ Celsius T = TC + 273.15 5 Example (text problem 13.3): (a) At what temperature (if any) does the numerical value of Celsius degrees equal the numerical value of Fahrenheit degrees? TF = 1.8TC + 32 = TC TC = −40 °C (b) At what temperature (if any) does the numerical value of Kelvin equal the numerical value of Fahrenheit degrees? TF = 1.8TC + 32 = 1.8(T − 273) + 32 = 1.8(TF − 273) + 32 TF = 574 °F 6 §13.3 Thermal Expansion of Solids and Liquids Most objects expand when their temperature increases. 7 An object’s length after its temperature has changed is L = (1 + αΔT )L0 α is the coefficient of thermal expansion where ΔT=T-T0 and L0 is the length of the object at a temperature T0. 8 How does the area of an object change when its temperature changes? The blue square has an area of L02. L0 L0+ΔL With a temperature change ΔT each side of the square will have a length change of ΔL = αΔTL0. 9 The fractional change in area is: new area = A = (L0 + αΔTL0 )(L0 + αΔTL0 ) = L + 2αΔTL + α ΔT L 2 0 2 0 2 2 2 0 ≈ L20 + 2αΔTL20 = A0 (1 + 2αΔT ) ΔA = 2αΔT A0 10 The fractional change in volume due to a temperature change is: ΔV = βΔT V0 For solids β=3α 11 §13.4 Molecular Picture of a Gas The number density of particles is N/V where N is the total number of particles contained in a volume V. If a sample contains a single element, the number of particles in the sample is N = M/m. N is the total mass of the sample (M) divided by the mass per particle (m). 12 One mole of a substance contains the same number of particles as there are atoms in 12 grams of 12C. The number of atoms in 12 grams of 12C is Avogadro’s number. N A = 6.022 × 10 23 mol-1 13 A carbon-12 atom by definition has a mass of exactly 12 atomic mass units (12 u). This is the conversion factor between the atomic mass unit and kg (1 u = 1.66×10-27 kg). NA and the mole are defined so that a 1 gram sample of a substance with an atomic mass of 1 u contains exactly NA particles. 14 Example (text problem 13.39): Air at room temperature and atmospheric pressure has a mass density of 1.2 kg/m3. The average molecular mass of air is 29.0 u. How many air molecules are there in 1.0 cm3 of air? total mass of air in 1.0 cm 3 number of particles = average mass per air molecule The total mass of air in the given volume is: ⎛ 1.2 kg ⎞⎛ 1.0 cm ⎜ m = ρV = ⎜ 3 ⎟⎜ ⎝ m ⎠⎝ 1 3 ⎞⎛ 1 m ⎞ ⎟⎟⎜ ⎟ ⎠⎝ 100 cm ⎠ 3 = 1.2 ×10 −6 kg 15 Example continued: total mass of air in 1.0 cm 3 number of particles = average mass per air molecule 1.2 ×10 −6 kg = (29.0 u/particle) 1.66 ×10−27 kg/u ( ) = 2.5 ×1019 particles 16 §13.5 Absolute Temperature and the Ideal Gas Law Experiments done on dilute gases (a gas where interactions between molecules can be ignored) show that: For constant pressure V ∝T Charles’ Law For constant volume P ∝T Gay-Lussac’s Law 17 For constant temperature 1 P∝ V For constant pressure and temperature V∝N Boyle’s Law Avogadro’s Law 18 Putting all of these statements together gives the ideal gas law (microscopic form): PV = NkT k = 1.38×10-23 J/K is Boltzmann’s constant The ideal gas law can also be written as (macroscopic form): PV = nRT R = NAk= 8.31 J/K/mole is the universal gas constant and n is the number of moles. 19 Example (text problem 13.41): A cylinder in a car engine takes Vi = 4.50×10-2 m3 of air into the chamber at 30 °C and at atmospheric pressure. The piston then compresses the air to one-ninth of the original volume and to 20.0 times the original pressure. What is the new temperature of the air? Here, Vf = Vi/9, Pf = 20.0Pi, and Ti = 30 °C = 303 K. PiVi = NkTi Pf V f = NkT f The ideal gas law holds for each set of parameters (before compression and after compression). 20 Example continued: Take the ratio: Pf V f PiVi = NkT f NkTi = Tf Ti ⎛ Pf The final temperature is T f = ⎜⎜ ⎝ Pi ⎞⎛ V f ⎞ ⎟⎟⎜⎜ ⎟⎟Ti ⎠⎝ Vi ⎠ ⎛ Vi ⎞ ⎛ 20.0 Pi ⎞⎜ 9 ⎟ ⎟⎟⎜ = ⎜⎜ ⎟(303 K ) = 673 K ⎝ Pi ⎠⎜ Vi ⎟ ⎝ ⎠ The final temperature is 673 K = 400 °C. 21 §13.6 Kinetic Theory of the Ideal Gas An ideal gas is a dilute gas where the particles act as point particles with no interactions except for elastic collisions. 22 Gas particles have random motions. Each time a particle collides with the walls of its container there is a force exerted on the wall. The force per unit area on the wall is equal to the pressure in the gas. The pressure will depend on: •The number of gas particles •Frequency of collisions with the walls •Amount of momentum transferred during each collision 23 The pressure in the gas is 2N P= K tr 3V Where <Ktr> is the average translational kinetic energy of the gas particles; it depends on the temperature of the gas. K tr 3 = kT 2 24 The average kinetic energy also depends on the rms speed of the gas K tr 1 1 2 2 = m v = mvrms 2 2 where the rms speed is 3 1 2 kT = mvrms 2 2 3kT vrms = m K tr = 25 The distribution of speeds in a gas is given by the MaxwellBoltzmann Distribution. 26 Example (text problem 13.60): What is the temperature of an ideal gas whose molecules have an average translational kinetic energy of 3.20×10-20 J? 3 K tr = kT 2 2 K tr T= = 1550 K 3k 27 Example (text problem 13.70): What are the rms speeds of helium atoms, and nitrogen, hydrogen, and oxygen molecules at 25 °C? vrms = 3kT m On the Kelvin scale T = 25 °C = 298 K. Element Mass (kg) rms speed (m/s) He 6.64×10-27 1360 H2 3.32 ×10-27 1930 N2 4.64 ×10-26 515 O2 5.32 ×10-26 482 28 §13.7 Temperature and Reaction Rates For a chemical reaction to proceed, the reactants must have a minimum amount of kinetic energy called activation energy (Ea). 29 If 3 Ea >> kT 2 then only molecules in the high speed tail of MaxwellBoltzmann distribution can react. When this is the situation, the reaction rates are an exponential function of T. reaction rates ∝ e −⎛⎜ ⎝ Ea ⎞ kT ⎟⎠ 30 Example (text problem 13.76): The reaction rate for the hydrolysis of benzoyl-l-arginine amide by trypsin at 10.0 °C is 1.878 times faster than at 5.0 °C. Assuming that the reaction rate is exponential, what is the activation energy? r1 ∝ e r2 ∝ e −⎛⎜ ⎝ Ea −⎛⎜ ⎝ Ea ⎞ kT 1 ⎟⎠ ⎞ kT 2 ⎟⎠ where T1 = 10.0 °C = 283 K and T2 = 5 °C = 278 K; and r1 = 2.878 r2. ⎛ Ea Ea ⎞ r1 ⎟⎟ The ratio of the reaction rates is = exp⎜⎜ − + r2 ⎝ kT1 kT2 ⎠ 31 Example continued: Solving for the activation energy gives: ⎛ r1 ⎞ k ln⎜⎜ ⎟⎟ r2 ⎠ ⎝ Ea = ⎛1 1⎞ ⎜⎜ − ⎟⎟ ⎝ T2 T1 ⎠ ( 1.38 × 10 = ) J/K ln (1.878) = 1.37 × 10 −19 J 1 ⎞ ⎛ 1 − ⎜ ⎟ 278 K 283 K ⎠ ⎝ − 23 32 §13.8 Collisions Between Gas Molecules On average, a gas particle will be able to travel a distance Λ= 1 2πd 2 ( N / V ) before colliding with another particle. This is the mean free path. The quantity πd2 is the cross-sectional area of the particle. 33 After a collision, the molecules involved will have their direction of travel changed. Successive collisions produce a random walk trajectory. 34 Substances will move from areas of high concentration to areas of lower concentration. This process is called diffusion. In a time t, the rms displacement in one direction is: xrms = 2 Dt D is the diffusion constant (see table 13.3). 35 Example (text problem 13.81): Estimate the time it takes a sucrose molecule to move 5.00 mm in one direction by diffusion in water. Assume there is no current in the water. xrms = 2 Dt Solve for t ( ( −3 ) 2 x 5.00 × 10 m = = 25000 s t= −10 2 2D 2 5.0 × 10 m /s 2 rms ) 36 Summary •Definition of Temperature •Temperature Scales (Celsius, Fahrenheit, Absolute) •Thermal Expansion •Origin of Pressure in a Gas •Ideal Gas Law •Exponential Reaction Rates •Mean Free Path 37