Respiratory System By Dr. Carmen Rexach Physiology
advertisement

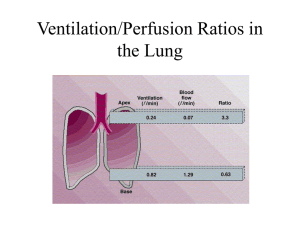
Respiratory System By Dr. Carmen Rexach Physiology Mt San Antonio College External vs. internal respiration • External respiration – ventilation – gas exchange • Internal respiration – cellular respiration Structure • Conducting zone – Nasal cavity to respiratory bronchioles • Respiratory zone – Respiratory bronchi – Alveoli Thoracic cavity • Diaphragm • Pleura • Potential space Intrapulmonary and intrapleural pressures • Boyle’s law – pressure of a given quantity of gas is inversely proportional to volume • Interpleural space = intrapleural space • Intrapulmonary (intraalveolar) pressure – Pressure in alveoli • Intrapleural pressure – Pressure in pleural cavity • Transpulmonary pressure – Intrapleural pressure – intrapulmonary pressure – Keeps lungs inflated Relationship between intrapulmonary and intrapleural pressure Pneumothorax • Air in interpleural space is below atm • When wall is breached, air rushes in – GSW, stabbing, trauma • Result: collapsed lung Spontaneous pneumothorax • Lung collapses due to air or gas collecting in chest without any sign of traumatic injury • Usually occurs when patient is resting • Symptoms – Sudden chest pain with breathlessness, exaccerbated with deep breathing or coughing • Risk factors – Male gender (7x’s more likely than in females) • Smoking (22x’s more likely than nonsmokers) – Smoking females 9x’s more likely than nonsmoking females Inspiration • Pressure of air exceeds intrapulmonary pressure • Steps: – expansion of thoracic cage – pulls on parietal pleura = increase intrapleural cavity volume – pressure decreased by (subatmospheric) – increased transpulmonary difference – alveoli expand = decreased pressure in alveoli – air moves from high to low pressure = moves in Expiration • Intrapulmonary pressure greater than atmospheric pressure = air moves out • Steps: – diaphragm & inspiratory intercostals relax – chest wall recoils – intrapleural pressure approaches preinspirational value – intrapulmonary pressure exceeds atmospheric pressure – air goes out Physical properties of the lungs • Three properties – Compliance – Elasticity= tendency to recoil – Surface tension • Two forces resist distension – Surface tension and recoil • surfactant Pulmonary ventilation • Normal inspiration = active • Normal expiration = passive • Forced inspiration – Scalenes, pectoralis major, sternocleidomastoid • Forced expiration – Internal intercostals,abdominals Pulmonary function tests • Measured by spirometry • Lung volumes and capacities (approximate volume) – Tidal volume = volume of each breath (500ml) – Vital capacity = largest possible tidal volume; amount of gas that can be forcefully exhaled after maximum inhalation (5000ml) – Inspiratory reserve volume = volume of gas that can be forcefully inhaled after a normal inhalation (3000ml) – Expiratory reserve volume = volume of gas that can be forcefully exhaled after an unforced exhalation (1500ml) – Residual volume = amount of gas remaining in the lungs after a forced expiration (100ml) – Dead space volume = volume of air in the conduction passageways that is not exchanged (150ml) Differences by gender Pulmonary disorders • • • • Dyspnea Asthma Emphysema COPD =chronic bronchitis + emphysema • Pulmonary fibrosis bronchi Normal lung Chronic bronchitis asthma Emphysema alveoli Partial pressure of gases • Dalton’s law • PN2 + PO2 + PCO2+ PH2O = PATM = 760mmHg • air = 21% O + 78% N 0.21 760 159mm Hg 0.78 760 593mm Hg 0.0004 760 0.3mm Hg Other factors influencing pressure • Altitude – Increased = decreased atmospheric pressure – Decreased = increased atmospheric pressure • 1 atm increase for every 33 feet below sea level • Temperature – determinant of water vapor composition of air – in body • water vapor = 47mm Hg • effects the partial pressure of O2 = 105 mm Hg in alveoli Partial pressure of gases in the blood • Gases diffuse quickly due to: – surface area, large capillary bed, short diffusion distance • Henry’s law = The maximum value of a gas dissolved in a fluid depends on: – the solubility of the gas in fluid – temperature of the fluid – partial pressure of the gases • Oxygen content of the blood depends on – PO2, # of RBC’s, hemoglobin content – Remember: Oxygen is primarily bound to Hb in RBC’s keeping the amount of O2 in the plasma low How oxygen is carried in the blood • Normal resting oxygen consumption = 250ml/min • PO2 = 100mm Hg in PV = 20ml O2/100 ml blood – 0.3ml O2 dissolved in plasma – 19.7ml O2 in RBC’s Partial pressure of CO2 and O2 in circulation Vascular resistance in lungs • Vascular resistance – fetal = collapsed lungs, resistance is high – birth = drops • subatmospheric intrapulmonary pressure opens blood vessels • stretching of lungs at inspiration • dilation of pulmonary arterioles due to increased alveolar PO2 • foramen ovale and ductus arteriosus close – adult = low pressure/low resistance • blood flows to lungs and to systemic circulation at same rate • pulmonary 1/10th of systemic vascular resistance Ventilation/perfusion ratios (V/P) – Ventilation = respiration rate x tidal volume – Perfusion = pulmonary blood flow = heart rate x right ventricular SV – Nearly matched under normal conditions • apex of lung – overventilated & underperfused – apex =3.4:1 – larger alveoli • base of lung – underventilated & overperfused – base = 0.6:1 Disorders caused by high partial pressures of gases • Oxygen toxicity – PO2 > 2.5 atm – oxidation of enzymes, nervous system damage, coma, death • Nitrogen narcosis – > one hour down – rapture of the deep, drowsiness, “intoxication” • Decompression sickness – formation of N2 bubbles in blood – channels blocked, joint & muscle pain = the bends Hyperbaric oxygen therapy • 100% oxygen at >1atm (US = 2.0-2.4 atm abs) • Duration:60-90 min. • Result: Arterial PO2 = 1200mmHg • Benefits: – Enhanced fibroblast replication – Activation of osteoclasts – Stimulation of capillary growth – Upregulation vascular endothelial growth factor – Upregulation of platelet derived growth factor CID: 2006 (43):188-192 Hyperbaric treatment for diabetic foot ulcers 40 days after hyperbaric treatment & skin graft Before hyperbaric treatment Brain stem respiratory centers • Medulla oblongata – rythmicity center • dorsal group (phrenic nerve) & ventral group (intercostals) • I neurons = inspiration = spinal motor neurons innervate respiratory muscle • E neurons = fire during expiration and inhibit I neurons • Pons – apneustic center -- constant I neuron stimulation – pneumotaxic center -- inhibitory = cyclic inhibition • Chemoreceptors -- respond to changes in PCO2, pH, PO2 – central – peripheral = aortic and carotid bodies Irritant and Inflation Reflex • Pulmonary irritant reflexes – Reflex constriction to prevent particulates from entering lungs – Stimulate cough in trachea & bronchi, sneeze in nasal cavity • Inflation reflex – Stretch receptors respond to lung inflation – Inhibitory signals sent to allow expiration to occur – Hering-Breuer Reflex Control of ventilation: blood CO2 • Chemoreceptors control rate & depth of breathing by measuring PCO2, PO2, pH – Hypoventilation = hypercapnia – Hyperventilation = hypocapnia • reflex control of ventilation – goal: to maintain relatively constant PCO2 = 40 mm Hg • chemoreceptors in ventral medulla – – – – – increased arterial PCO2 = inc [H+] blood CSF = CO2 crosses blood blain barrier to stimulate receptors Periphery = rise in [H+] decreases blood pH = stimulus In the brain, CO2 levels directly stimulate receptors in the periphery, H+ levels provide the stimulus Peripheral chemoreceptors Effects of blood PO2 on ventilation • Indirect influence by changing chemoreceptor sensitivity to CO2 – low PO2 = increased sensitivity – high PO2 = decreased sensitivity • effect of breathing pure oxygen – dilutes effect of CO2 • Chronic CO2 exposure – diminished response (emphysema) Hemoglobin Hemoglobin • 2 α & 2 β chains = quaternary structure • 4 hemes = each heme has one Fe and will bind with one oxygen molecule • 280 million Hb per RBC x 4 = >1 billion molecules of oxygen per RBC • Hb + O2 = oxyhemoglobin • Hb - O2 = deoxyhemoglobin • oxygen saturation = statistical average of all oxygen bound relative to total amount that can be bound What binds to hemoglobin? • • • • • oxyhemoglobin = Hb + O2 deoxyhemoglobin = Hb - O2 carbaminohemoglobin = Hb + CO2 carboxyhemoglobin = Hb + CO methemoglobin = Fe3+ instead of Fe2+ – cannot bind oxygen – normally represents 1-2% of Hb • Sulfhemoglobin = Hb + Sulfur Unusual conditions • Sulfhemoglobinemia – Increased amounts of sulfur, usually drug induced – Blood is green due to binding of sulfur to Hb • Methemoglobinemia – – – – Increased amount of Fe3+ on Hb Blood appears chocolate brown in color Patients look “blue” NOTE: Venous blood is not blue in normal people!! It just looks blue through skin because veins run deeper than arteries Hemoglobin concentration • oxygen carrying capacity of the blood = maximum amount that can be bound by Hb • <normal =anemia • >normal = polycythemia (common at high altitudes) • RBC/Hb production – erythropoietin – androgens Properties of Hb:O2 binding • Hb binds reversibly with O2 • Molecular oxygen associates and dissociates from Hb very rapidly – Blood is in the exchange capillaries less than one second • The sigmoid shape of the oxyhemoglobin dissociation curve is caused by molecular interactions of the four heme groups Loading and unloading reactions • Loading reaction • Unloading reaction • Determined by: – PO2 of the environment – Affinity of Hb for oxygen Oxyhemoglobin dissociation curve • Relationship between PO2 and oxygen saturation of Hb • Oxygen reserve – 80% saturation even at PO2 of 40 mm Hg • Effects of high PO2 • Can be modified by physiological and pathological factors – pH – temperature – 2,3-DPG Oxyhemoglobin dissociation curve Effect of pH, temperature, &2,3 DPG on Oxygen transport • incr [H+], PCO2, 2,3-DPG, temperature = decr affinity of Hb for oxygen = incr unloading – entire curve shifts to the right of the standard curve • decr [H+], PCO2, 2,3-DPG, temperature = incr affinity of Hb for oxygen = incr loading – entire curve shifts to the left of the standard curve 2,3-DPG (diphosphoglyceric acid) • Product of anaerobic respiration in RBC’s • increases with decrease in oxyhemoglobin • result: increased unloading of oxygen at tissues • conditions – anemia – high altitudes – transfer maternal to fetal circulation (Hbf) Shifts in oxyhemoglobin dissociation curve Inherited defects in hemoglobin structure/function • Sickle cell anemia (HbS) – valine replaces glutamic acid on β chain • thalassemia – Mediterranean ancestry – 2 forms; α & β thalassemia – increased γ chain production, decreased oxygen unloading Muscle myoglobin • Special functions – middleman – oxygen storage function • Slow twitch fibers & cardiac muscle cells • rhabdomyolysis How is CO2 carried in blood? • 1/10 = dissolved • 1/5 = carbaminohemoglobin • 7/10 = bicarbonate – CO2 + H20 H2CO3 • Carbonic anhydrase – in RBC’s H+ + HCO3- Chloride shift: tissue level • Equation shifts to the right – H2O + CO2 • Steps: H2CO3 H+ + HCO3- – CO2 diffuses out of the tissue cells into the blood – CO2 moved into the red blood cells – Combines with H2O in the presence of carbonic anhydrase to produce carbonic acid – Carbonic acid dissociates producing H+ + HCO3– H+ buffered by hemoglobin, facilitating the offloading of O2 – net positive charge in RBC results in chloride shift – Chloride moves into the RBC in exchange for HCO3– Bohr effect • increased oxygen unloading • continued H2CO3 production • enhanced transport of CO2 Chloride Shift: Tissue Level Chloride shift: Pulmonary capillaries • Hb oxygenated • decrease in affinity for H+ • Reverse chloride shift as Cl- moves out and HCO3- moves in • HCO3- + H+ H2CO3 • Carbonic acid dissociates to CO2 & H2O • CO2 expired out • Remember: – H+ is buffered by Hb in RBC – HCO3- goes into the plasma and buffers incoming H+ Reverse Chloride Shift Ventilation and acid-base balance • Acidosis and alkalosis • Regulated by respiratory system – Respiratory acidosis – Respiratory alkalosis • Regulated by the kidneys – Metabolic acidosis – Metabolic alkalosis Ventilation during exercise • Neurogenic – sensory nerve activity = stimulates respiratory muscles – cerebral cortex = brain stem alteration of ventilation • humoral – cyclic variations in values of PCO2 & pH stimulates chemoreceptors (small amounts) • anaerobic threshold and endurance training – anaerobic threshold = maximum rate of oxygen consumption attained before blood lactic acid levels rise due to anaerobic respiration – adaptations in athletes =incr mitochondria, aerobic enzymes; incr oxygen utilization by muscles, lower % oxyhemoglobin in venous blood Higher altitudes • Conditions differ – rapid fatigue: decreased PO2, oxygen content of blood decreased (PO2 =69-74mmHg, oxyhemoglobin saturation = 9293%) • Changes in ventilation – hypoxic ventilatory response: decr arterial PO2 = hyperventilation = respiratory alkalosis • mediated by incr in pH, stabilizes after a few days – cannot increase PO2 greater than inspired air • Hemoglobin affinity for oxygen decreased – greater unloading due to 2,3-DPG • Hemoglobin and RBC production – tissue hypoxia stimulates increased erythropoietin – increased viscosity due to increase in RBC’s