Document 10288544
advertisement

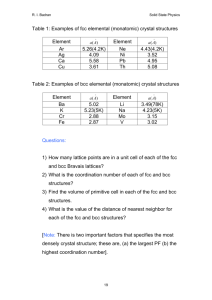
MSE 200A Survey of Materials Science Fall, 2008, Problem Set No. 1 Problem 1: In the diamond modification of solid carbon each atom has exactly four nearest neighbors that are configured in a tetrahedral coordination around it. In the graphite modification of carbon the carbon atoms form planar sheets that are relatively widely separated from one another. Each carbon atom is bonded to three near neighbors in the plane, and has a weak interaction with atoms in adjacent planes. (a) Diamond is an electrical insulator and is one of the hardest substances known. Interpret these properties in terms of its bonding. (b) Graphite is an anisotropic conductor. It has high conductivity in the plane of the sheet, and very low conductivity perpendicular to the sheet plane. Explain this behavior in terms of a simple chemical bonding model. (c) In its usual microstructure, small three-dimensional particles made of stacked planar sheets, graphite deforms easily, and is used in pencil lead and lubricants. Given that the carbon sheets themselves are very rigid, explain why graphite is so easily deformed. (d) Graphite fiber is made by modifying the microstructure into long, thin ribbons in which the carbon sheets parallel the plane of the ribbon. The ribbons are then rolled, somewhat as one would roll a newspaper, to form the graphite fiber. While normal graphite is weak, graphite fiber is extremely strong when pulled along the fiber axis. Explain in terms of the microstructure and the bonding in graphite. Problem 2: Let two atoms bond together so that the potential energy of the pair is described by equation 2.1 of the notes. (a) Find the equilibrium separation of the atoms, r0, in terms of the constants A, B, m and n. (b) Show that the molecule behaves like a simple spring for small displacements, ∂r = r - r0, away from the equilibrium separation; that is, show that the force that acts to restore the equilibrium separation is given approximately by the relation F = - K∂r Evaluate the spring constant, K. Page 1 MSE200A: Fall, 2008 Problem Set No. 1 (c) Find the force that would have to be applied to the molecule to break it in two. This is the ultimate tensile strength of the molecule. Problem 3: (a) Consider a two-dimensional crystal made up of spherical atoms that are packed together as tightly as possible in the plane. Draw the Bravais lattice for this crystal, and the primitive cell defined by the unit vectors of the Bravais lattice. (b) Show that the unit cell can be drawn as a hexagon with an atom in the center and atoms at each of the six corners. How many atoms are there per cell? (c) Consider a two-dimensional crystal that has a hexagonal unit cell with an empty center and atoms located at the corners of the hexagon. Draw the atom positions generated by several neighboring cells. Show that the unit cell used in part (b) is a satisfactory cell for this structure, but the cell used in part (a) is not. (d) Draw a proper set of lattice vectors for the structure in (c). Indicate the basis vectors that are needed to complete the description of the structure. Given your choice of lattice vectors, what atom group is repeated at each lattice point? Problem 4: (a) Prove that there is a [–101] direction in the (111) plane. (b) Prove that there is no [101] direction in the (111) plane. (c) Find the angle at which the (111) and (–111) planes intersect. [Hint: this is a very difficult problems in geometry unless you remember that the [hkl] direction is a vector perpendicular to the (hkl) plane and recall that the scalar product of two vectors, a and b, obeys the relation a^b = |a||b| cos(œ) With this relation the problem becomes simple.] Problem 5: (a) Let the lattice sites of the FCC bravais lattice be filled with spherical atoms that are close-packed in the sense that they just touch one another. What is the radius of the largest sphere that will fit inside an octahedral void without distorting the structure? What is the radius of the largest sphere that will fit in a tetrahedral void without distortion? [Hint: the geometry of the tetrahedral void is much easier to treat if one first shows that the center of the void lies at the center of a cube that is 1/8 the size of the FCC unit cell.] (b) Let a crystal have the hexagonal close-packed structure, and consist of spherical atoms that just touch one another. Find the number of octahedral and tetrahedral voids per atom, and compute the sizes of the largest spheres that will fit in each type of site without distortion in terms of the radius, R, of the atoms on the HCP lattice sites. [Hint: assuming that you have solved parts (a) and (b), if you have to compute anything at all to answerthis question you are approaching it wrong.] page 2 MSE200A: Fall, 2008 Problem Set No. 1 Problem 6: (a) Let the lattice sites of the BCC bravais lattice be filled with spherical atoms that are close-packed in the sense that they just touch one another. What is the radius of the largest sphere that will fit inside an “octahedral” void without distorting the structure? What is the radius of the largest sphere that will fit in a “tetrahedral” void without distortion? (b) The common interstitial solutes in BCC metals, in particular, C and N in Fe, are found in the “octahedral” interstices rather than the tetrahedral ones. Given your answer to part (a), how do you explain this? Problem 7: (a) The perovskite crystal structure, which is a common structure of ferroelectric oxides like BaTiO3, is usually drawn in a cubic cell with Ti atoms at the corners, Ba at the center, and O at the centers of each of the edges. Draw the structure and show that it has the stoichiometric formula BaTiO3.. (b) To relate this structure to FCC most simply we consider lattice vacancies as a component. By placing a vacancy at the center of each face of the cube show that BaTiO3 can be regarded as the compound (Ti 3)(BaO3) where is a vacancy. The FCC lattice is filled by Ti and vacancies while the octahedral interstitial voids are filled by Ba and O. Show that this structure is the NaCl structure if we ignore the difference between Ti and , and between Ba and O. Show that Ti and are distributed over the FCC sites in the Cu3Au pattern, while Ba and O are distributed over the FCC lattice of octahedral voids in a similar pattern. (c) Show that the perovskite structure of part (a) can also be understood as a BCC lattice filled by Ba and Ti with O atoms in one-half of the octahedral voids. Show that the Ba and Ti atoms are substitutionally ordered in a CsCl pattern. (d) While this representation may seem to be a simpler description of the structure, in at least one important respect it is not. Show that the O atoms do not have a simple BCC pattern, even when vacancies are included. [To treat the structure as a set of BCC sublattices with CsCl order on all of them one must assume that the oxygen is distributed over three separate sublattices, one associated with each type of octahedral void (Ox, Oy, and Oz). The mixture of oxygen and vacancies on each of the oxygen sublattices is ordered in a CsCl pattern. However, this leads to a much more complicated picture of the structure.] page 3