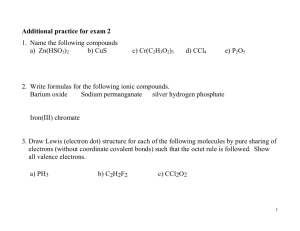
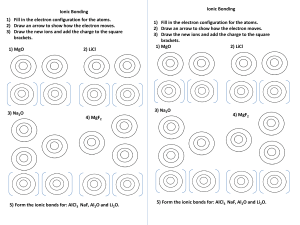
Review Topics: Chapter 3 CHEM 1A

Review Topics: Chapter 3
CHEM 1A
Covered in Lecture:
Ion formation and size o Noble gas electron configurations o Metals versus non-metals o Gain and loss of electrons
which electrons are lost first o Size relative to neutral parent atom
attractive force between positive nucleus and negative electrons
electron – electron repulsion
Periodic trends o Atomic radii (covered in Chapter 2, section 2.14) o Ionization energy
relative magnitudes of subsequent ionization energies
dips in periodic trends o Electron affinity o Electronegativity o Metallic behavior o Acidity of oxides o Acidity of nonmetal hydrides
Chemical bonding o Interacting atoms: why bonds form o Electronegativity and types of bonds o Approximate difference in electronegativity cutoffs
Ionic bonding o Diff. EN > 1.7 o How the ions are held together in the solid o Properties of ionic solids
melting points
hardness
cleavage
conductivity o Lattice energy (
H lattice
)
Coulomb’s law (charges and distance)
predicting relative lattice energies for various ionic compounds
effect of lattice energy on melting point, hardness, and solubility in water
Page 1 of 2
Covered in Lab (Nomenclature Worksheet):
Formation of ions and common ionic charges o Using the periodic table to predict the ionic charges of representative (main group) elements and select other elements
Nomenclature o Ionic compounds
simple binary ionic compounds
monovalent and polyvalent metals and the stock system
polyatomic ions and oxyanions
Manage your time wisely as you study. Cover all the basic concepts before delving too deeply into any one topic. If you have a specific question, you can e-mail me at albi.romero@cabrillo.edu
I will reply to e-mails several times the night before an exam. Continue to study other topics while you wait for a response.
Page 2 of 2