Chapter 21: Carboxylic acid Derivatives I. Introduction
advertisement

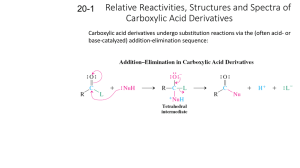
1 Chapter 21: Carboxylic acid Derivatives I. Introduction O R C X O O R C O C R O R C O-R' O R C NH 2 R C N II. Nomenclature of Carboxylic Acid Derivatives A) Esters Cyclic Esters Cyclic esters are called lactones. Names of lactones are derived by adding the term lactone at the end of the name of the parent carboxylic acid it came from: B. Amides of Carboxylic Acids © John Congleton 2 Nomenclature of Amides 1) Name the related carboxylic acid. 2) Replace the “-oic acid” (for IUPAC names) or the “-ic acid” suffix (for common names) with “-amide”. 3) If the are alkyl groups on the nitrogen, put the name of each alkyl group in front with an “N-” prefix (for example: N-ethyl). Examples: © John Congleton 3 Cyclic amides are known as Lactams. Lactams are produced from amino acids, where the amino and the carboxylic acid groups react together to form an amide linkage. C. Nitriles of Carboxylic Acids Nitriles are technically not derivatives of carboxylic acids, but they can be converted to carboxylic acids by hydrolysis, so they have been included in this chapter: Nomenclature of Nitriles 1) Count the number of carbons in the longest carbon chain that contains the nitrile (CN) carbon. Include the nitrile carbon in the count. 2) Name the alkane that has the same number of carbons. 3) Add the suffix “-nitrile” to the alkane name, with no space in between. D. Acid Halides Observed Reaction: (mechanism in chapter 20) © John Congleton 4 The halogen atom inductively withdraws electron density away from the already electrophilic carbon of the carbonyl group. Nomenclature of Acid Halides 1) Name the related carboxylic acid. 2) Replace the “-ic acid” suffix with “-yl halide”. E. Anhydrides of Carboxylic Acids The word anhydride literally means without water, and an acid anhydride is the combination of two molecules of carboxylic acid with the elimination of one molecule of water. Nomenclature of Acid Anhydrides To name acid anhydrides: 1) Name each carboxylic acid half, then drop the “acid” part of the names. 2) Put the names in alphabetical order, separated by a space. 3) Add the word “anhydride”, separated by a space, following the names. 4) If the two halves are the same, only write the name once. © John Congleton 5 F. Nomenclature of Multifunctional Compounds We have now covered a wide variety of functional groups. Many molecules contain more than one of these different functionalities. In choosing the principal group for the root name, the following priorities are observed. III. Interconversion of Acid Derivatives There are a lot of transformations, but only one general mechanism: © John Congleton 6 A. Reactivity of Acid Derivatives IV. INTERCONVERSION OF ACID DERIVATIVES A) Mechanism(s) of acid chloride reactions Acid chloride to an anhydride Acid chloride to ester © John Congleton 7 Acid chloride to amide B. Anhydrides Anhydride to an ester Anhydride to an Amide C. Esters Ester to an Amide © John Congleton 8 V. Leaving groups in Nucleophilic Acyl Substitutions CASE #1: SN2 CASE #2: Acyl Substitution © John Congleton 9 VI.Transesterification Ester + alcohol (in the presence of a strong acid) = transesterification This reaction can proceed either by the acid catalyzed mechanism or by the base catalyzed route. CASE #1 Acid Catalyzed: CASE #2 Base Catalyzed © John Congleton 10 VII. Hydrolysis of Carboxylic Acids Derivatives A) Hydrolysis of Acid Halides and Anhydrides Acid halides are more reactive than anhydrides. Acid anhydrides are less reactive and can be handled in the atmosphere for brief periods without a large amount of hydrolysis. Acid Chlorides are not stable in water. They react instantly with water. Even moisture in the air will cause reaction when they are exposed to the atmosphere. 1. Hydrolysis of acid chlorides (acid, base, or neutral solutions work) 2. Hydrolysis of Anhydrides (acid, base, or neutral solutions work) B. Hydrolysis of Esters CASE #1 The acid catalyzed hydrolysis of an ester is simply the reverse reaction of the Fischer esterification. © John Congleton 11 CASE #2 The basic hydrolysis of esters is also known as saponification, and this does not involve the equilibrium process observed for the Fischer esterification. © John Congleton 12 C. Hydrolysis of Amides CASE #1: Acidic conditions, the hydrolysis of an amide resembles the acid catalyzed hydrolysis of an ester, with protonation of the carbonyl group giving rise to an activated carbonyl group, that undergoes nucleophilic attack by water. CASE #2: (BASE)Amides are the most reluctant derivatives to undergo hydrolysis, but they can be forced to by the use of vigorous conditions such as heating with 6M HCl or 40% NaOH for prolonged periods of time. © John Congleton 13 D. Hydrolysis of Nitriles Nitriles are hydrolyzed to primary amides, and then on to carboxylic acids. Mild conditions only take this hydrolysis to the amide stage, and more vigorous conditions are required to convert the amide to a carboxylic acid. CASE #1: Acidic conditions Mechanism: Formation of the Amide and acid-catalyzed hydrolysis © John Congleton 14 CASE #2 Basic conditions VIII. Reduction of Acid Derivatives 1. Reduction to Alcohols Lithium aluminum hydride will reduce acids, acid chlorides and esters to primary alcohols (Ch 20). © John Congleton 15 Esters and acid chlorides react through the addition elimination mechanism, generating aldehydes, that are quickly reduced to the primary alcohols. 2. Reduction to Aldehydes 3. Reduction TO Amines Lithium aluminum hydride reduces amides, azides and nitriles to amines. © John Congleton 16 IX. Acid Derivatives and Organometallic Reagents Esters and acid chlorides will react twice with Grignard (and organolithium) reagents to produce alkoxides (Ch 10). Protonation of the alkoxides generates alcohols. Nitriles Organometallic reagents attack the electrophilic carbon of the nitrile group, and generate the metal salt of an imine. Acid hydrolysis of this salt not only protonates the salt to form an amine, but also hydrolyses the imine to a ketone (Ch 18). © John Congleton