Preparation and Properties of O and H
advertisement
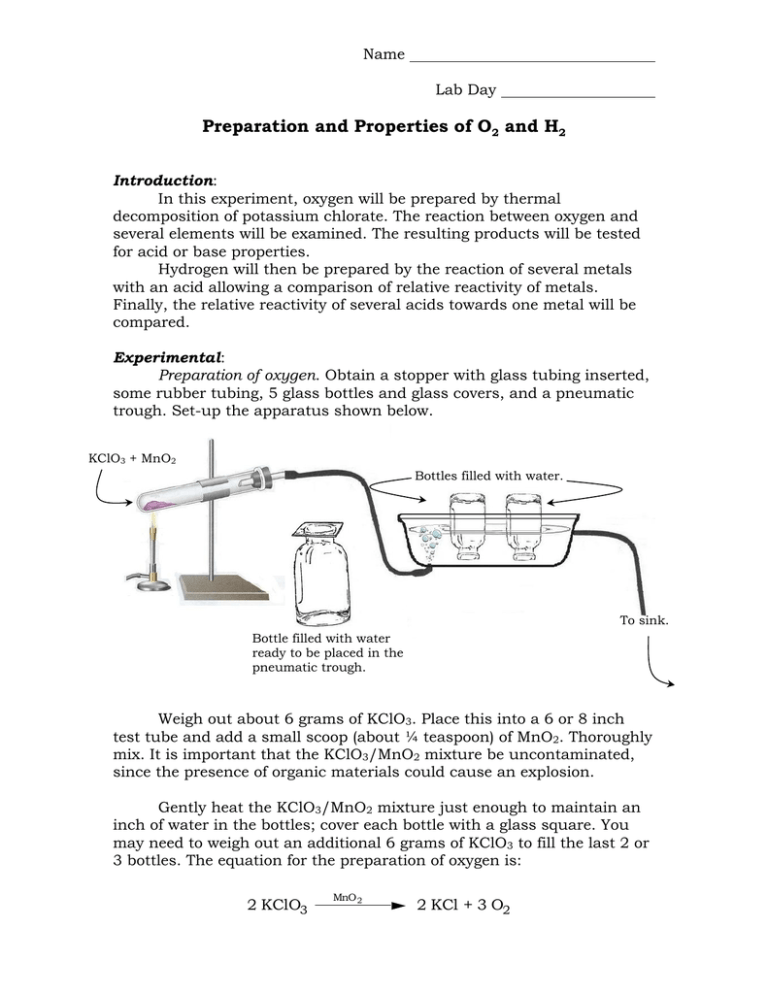
Name Lab Day Preparation and Properties of O2 and H2 Introduction: In this experiment, oxygen will be prepared by thermal decomposition of potassium chlorate. The reaction between oxygen and several elements will be examined. The resulting products will be tested for acid or base properties. Hydrogen will then be prepared by the reaction of several metals with an acid allowing a comparison of relative reactivity of metals. Finally, the relative reactivity of several acids towards one metal will be compared. Experimental: Preparation of oxygen. Obtain a stopper with glass tubing inserted, some rubber tubing, 5 glass bottles and glass covers, and a pneumatic trough. Set-up the apparatus shown below. KClO3 + MnO2 Bottles filled with water. To sink. Bottle filled with water ready to be placed in the pneumatic trough. Weigh out about 6 grams of KClO3. Place this into a 6 or 8 inch test tube and add a small scoop (about ¼ teaspoon) of MnO2. Thoroughly mix. It is important that the KClO3/MnO2 mixture be uncontaminated, since the presence of organic materials could cause an explosion. Gently heat the KClO3/MnO2 mixture just enough to maintain an inch of water in the bottles; cover each bottle with a glass square. You may need to weigh out an additional 6 grams of KClO3 to fill the last 2 or 3 bottles. The equation for the preparation of oxygen is: 2 KClO3 MnO 2 2 KCl + 3 O2 2 Preparation of oxides. Burn the following elements in oxygen gas using a combustion spoon (below left) or crucible tongs (below center), keeping the bottles covered with the glass as much as possible. Use the Fischer burner (below right) for all heating except the Mg & S. 1. 2. 3. 4. 5. Substance Magnesium – Ignite a piece of ribbon using the tongs shown below then thrust into a bottle. Avoid looking at the bright flame. Iron – Ignite a ball of steel wool using the tongs below. Carbon – Use a chunk of charcoal and the tongs below. Calcium – Grab a small piece using the tongs below and heat till red hot. Avoid looking at the bright flame. Sulfur – (In hood only!) Use a small scoop in combustion spoon. Observations Litmus Test* Magnesium Iron Carbon Calcium Sulfur *Test the resulting solutions with 1 drop of Litmus solution. Litmus turns red in acid, blue in base. 3 Experimental: Preparation of hydrogen. Place 5 mL of dilute (6 M) hydrochloric acid into each of 3 test tubes. Add a small piece of Al, Cu, and Pb. Allow the test tubes to sit for at least 10 minutes. Note any tubes in which a gas is evolving. Test the gas for hydrogen with a burning splint. Record your observations below: Metal Observations Hydrogen Test Aluminum Copper Lead Arrange the metals in order of reactivity towards hydrochloric acid. Most reactive , , Least Reactive Relative Reactivity of Acids. Place 5 mL of dilute hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), and acetic acid (HC2H3O2), into 4 separate test tubes. Into each of the four test tubes, place a small piece of zinc metal. Compare reactions. Test for the evolution of hydrogen gas with a burning splint. Record your observations below: Acid HCl H2SO4 HNO3 HC2H3O2 Hydrogen Test Arrange the acids in order of reactivity towards zinc. Most reactive , , , Least reactive





