meeting report
Exploring the pole: an EMBO conference on
centrosomes and spindle pole bodies
Sue L. Jaspersen and Tim Stearns
The centrosome and spindle pole body community gathered for its triennial meeting from 12–16 September, 2008 at EMBL in
Heidelberg (Germany).
Sponsored by the EMBO, the conference on
centrosomes and spindle pole bodies was
organized by Trisha Davis, Susan Dutcher,
Michael Knop, Robert Palazzo, Elmar Schiebel
and Kip Sluder. This was the fourth meeting
in a series that started in 1996 and, as with the
previous meetings1–3, was an occasion to celebrate present accomplishments and contemplate the future. Below we summarize some of
the major themes that emerged.
Centrosome 101
Microtubules and their constellation of associated proteins and structures are strongly
conserved components of all eukaryotic cells.
One of the universal themes in the microtubule
cytoskeleton is the use of specific structures to
organize microtubules into useful arrays. The
centrosome of animal cells and the spindle pole
body of fungi are the two best characterized
microtubule-organizing structures and were
the topic of this meeting. The centrosome
contains a pair of centrioles surrounded by
a matrix of proteins involved in microtubule
nucleation and other centrosome functions.
This matrix of proteins is usually referred to as
pericentriolar material (PCM), although many
of the components of PCM are also found at
other sites of microtubule organization in differentiated cell types.
Sue L. Jaspersen is at the Stowers Institute for
Medical Research, Kansas City, MO 64110 USA
and in the Department of Molecular and Integrative
Physiology, University of Kansas Medical Center,
Kansas City, KS 66160 USA. Tim Stearns is in the
Department of Biology, Stanford University, Stanford,
CA 94305 USA and in the Department of Genetics,
Stanford University School of Medicine, Stanford, CA
94305 USA. e-mail: slj@stowers-institute.org and
stearns@stanford.edu
Centrioles are short, cylindrical structures in
which the walls of the cylinder are made up of
nine specialized triplet microtubules. This elegant
nine-fold symmetry is absolutely conserved and
gives centrioles their characteristic ‘pinwheel’
appearance in cross-section. Separate from their
role as a focus of PCM, centrioles also nucleate the
ciliary axoneme, imparting their nine-fold symmetry to this structure as well. A centriole at the
base of a cilium is referred to as a basal body.
The centrosome, with its pair of centrioles,
duplicates once per cell cycle at the G1/S transition so that a cell will have exactly two centrosomes during mitosis. Centrioles reproduce
semi-conservatively; the pairs separate and each
‘mother’ centriole grows a new ‘daughter’ centriole from its side. The centrosome has a mutualistic relationship with the mitotic spindle,
helping to form the poles of the spindle, while
at the same time using that spindle to segregate
equally to the sister cells of a division. This equal
segregation of one centrosome per cell ensures
that each cell has the potential to grow a cilium,
which is imparted by the mother centriole.
Most fungi have lost the capacity to make
centrioles and cilia but have evolved a morphologically distinct structure, known as the spindle
pole body (SPB), to serve as their primary site of
microtubule nucleation. The functional orthology of the SPB to the centrosome is reflected
in the conservation of some of the important
components, and genetic and biochemical analysis of SPBs have provided valuable insight into
centrosome regulation and function.
Centrosome parts
The rate-limiting step in understanding the
centrosome has been the definition of its
constituent proteins, and the identification
of those that are key functional components,
as opposed to hangers-on that use the centrosome as a cellular assembly point. At the first
meeting twelve years ago, John Kilmartin’s
mass-spectrometry analysis of the SPB4 was
a prescient first glimpse of the cornucopia of
centrosome proteins that would soon emerge
from similar work on centrosomes, centrioles
and cilia. Whereas we once had the sense of
having hold of only the trunk, leg or tail of the
proverbial centrosomal elephant, new results
are revealing a much more complete picture of
the organelle as a whole.
Jens Andersen described a refinement of the
original mass-spectrometry analysis of mammalian centrosomes5, using SILAC stable isotope
labelling technology to increase coverage and
specificity of results from impure centrosome
material. Jean Cohen presented a compilation
of centriole and cilia proteomic data from across
the eukaryotic world and an associated webbased analysis tool. In these and other proteomic
studies, the same proteins come up repeatedly,
suggesting that, by analogy to genetic screens,
we are close to saturation for identification of
new components. However, lest one become
sanguine about this prospect, Hannah Müller’s
proteomic analysis of centrosomes, isolated from
rapidly dividing Drosophila embryos, found only
limited overlap with the list of known centrosome proteins from other systems. Perhaps
this reflects differences in analysis techniques
or important biological changes in centrosome
components in the rapidly dividing embryo.
Genetic analysis has also made an important
contribution to identifying centrosome components and their interactions. The keynote
nature cell biology volume 10 | number 12 | DECEMBER 2008
© 2008 Macmillan Publishers Limited. All rights reserved.
1375
mee t i n g r e p o r t
SZY-20
Mps1
SCF
Cdk2-cyclinA/E
SPD-2
ZYG-1/Plk4
SAS-6
APC
SAS-6-P
SAS-5
SAS-4/CPAP
Bld10/Cep135
G1/S
cartwheel formation
centriole assembly
Separase
Plk1
S
centriole
elongation
M
centriole
disengagement,
cytokinesis
G2 and M
centriole
maturation &
separation
Bora
Plk1
Recruitment of PCM, appendage,
γ-TuRC, hPoc5, spindle pole proteins
Aurora-A
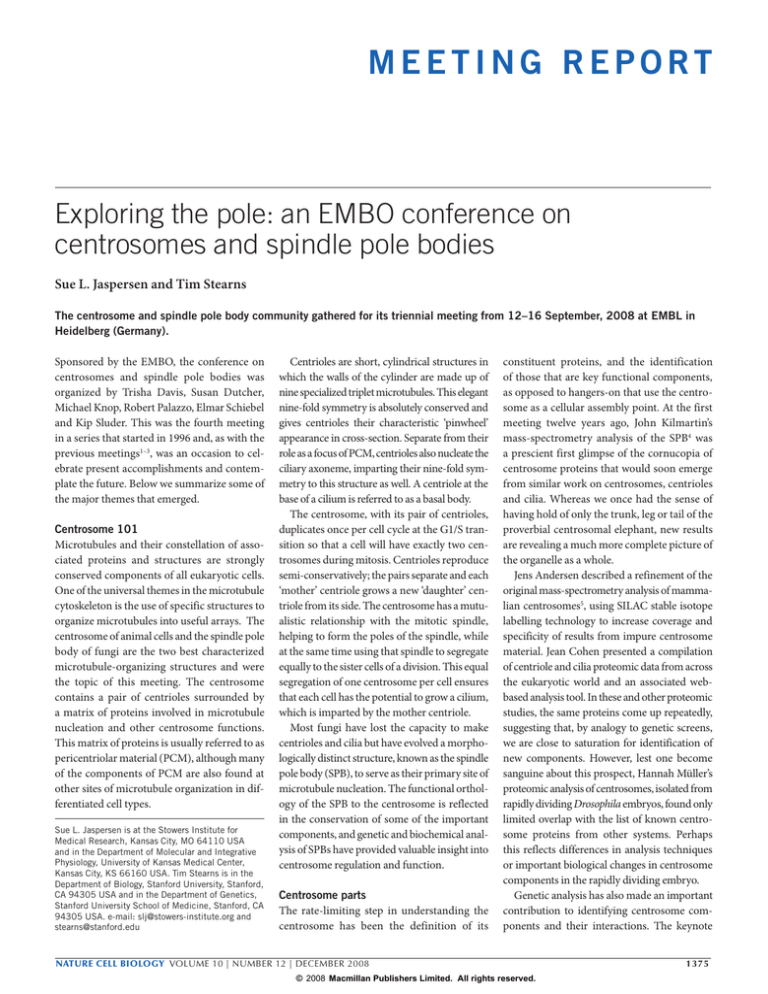
Figure 1 Centriole duplication pathway. Schematic representation of the major steps in centrosome duplication, as well as structural and regulatory proteins. We
have combined results from several systems, and the details may differ in specific systems (for reviews of the centriole cycle and its cell-cycle control, see refs 18,
29–31). At the end of mitosis, each of the two engaged centrioles within each pair become disengaged by the action of the separase protease and Plk1. The older
of the centrioles in each pair is marked with distal and sub-distal appendages, and the two centrioles remain linked by cohesion fibres. Centriole duplication is
initiated at the disengaged centrioles during G1/S by SPD-2, as well as the kinases Mps1 and Cdk2–cyclinA/E. A key regulatory step in centriole duplication is
activation of the kinase ZYG–1/Plk4; this involves control of its kinase activity, localization to the centrosome and, ultimately, proteolysis by the SCF ubiquitylation
complex. Active ZYG–1/Plk4 can phosphorylate SAS-6, a component of the cartwheel structure at the base of nascent centrioles. Recruitment of SAS-6,
SAS-5 and Bld10/Cep135 drive formation of the cartwheel, which imparts a nine-fold symmetry on the forming centriole. During S phase, centriole duplication
continues by recruitment of SAS-4/CPAP. Levels of CPAP are tightly controlled during the cell cycle by APC-mediated proteolysis, perhaps restricting centriole
assembly. Daughter centrioles continue to elongate during G2 and early M phase after activation of Aurora A at the centrosome, which is regulated by Plk1 and
Bora. The centrin-binding protein hPoc5 is also recruited to the centrosome. The new mother centriole matures by addition of components of distal and subdistal
appendages. The cohesion link between the two mother centrioles is broken, allowing the centrosomes to move to opposite sides of the nucleus. As mitosis
begins, γ-tubulin ring complex (γ-TuRC) and other PCM components are recruited to the centrosomes, along with mitotic spindle pole proteins. The two mitotic
centrosomes nucleate microtubules and help to form the mitotic spindle on which both chromosomes and centrosomes will segregate to the two daughter cells. At
the end of mitosis, separase and Plk1 trigger centriole disengagement, allowing the centrioles to duplicate in the G1/S phase, and completing the cycle.
address from Tony Hyman stressed the power
of RNA interference (RNAi) in Caenorhabditis
elegans and mammalian cells as a tool for identifying important components and determining
their function. Combining the ability to observe
early embryonic divisions of C. elegans with
whole-genome RNAi, Hyman and his worm­
ophile colleagues have discovered several of
the key players, including SAS-4 and SAS-6. He
described recent work revealing an unexpected
connection between centrosome size and spindle
length, which was independent of microtubule
nucleation. In addition, his group combined a
mammalian RNAi screen6 with tagging of proteins in BACs7 to identify interaction networks
among mammalian centrosome components.
1376
Similar RNAi screens have been performed
in Drosophila melanogaster cultured cells8,9.
Naomi Stevens described three genes, ana1,
ana2 and ana3, that are involved in centrosome
duplication and whose duplication results in
anastral spindles. Ana2 is structurally similar
to SAS-5, and like SAS-5 is required for centriole formation. Chad Pearson used basal body
proteome data from Tetrahymena10 to identify
Poc1 as a conserved centriole component that
is also required for centrosome duplication
and ciliogenesis in human cells. Using human
centrin as the bait in a yeast two-hybrid screen,
Michel Bornens identified hPoc5, the human
orthologue of a protein originally identified in
Chlamydomonas as a component of centrioles11.
Interestingly, hPoc5 contains Sfi1-like repeats,
which were originally discovered as Cdc31/centrin-binding motifs in the Saccharomyces cerevisiae SPB component Sfi1. Recruitment of hPoc5
to the distal lumen of centrioles, where centrin
2/3 are located, occurs late in G2 and involves
binding to its interacting partner, hPoc19, which
is recruited earlier in the cell cycle.
Centrosome pathways
One of the deeper mysteries of centrosome
biology is how the initiation of new centrioles
is controlled. As each centriole is potentially a
distinct centrosome, controlling initiation is
the key event in centrosome number control.
Although new centrioles typically grow from the
nature cell biology volume 10 | number 12 | DECEMBER 2008
© 2008 Macmillan Publishers Limited. All rights reserved.
mee t i n g r e p o r t
side of an existing centriole, Alexey Khodjakov’s
group has shown that generic mammalian cells
can produce centrioles de novo12, a property
they share with some single-cell organisms and
specialized mammalian cell types. However, the
presence of an existing centriole prevents this de
novo pathway. Khodjakov used laser ablation to
show that a mother centriole can only make a
new centriole after removal of its daughter. This
is consistent with experiments described by
Tim Stearns showing that in mammalian cells,
the protease separase and the kinase Plk1 act to
disengage the mother and daughter centrioles at
the end of mitosis, and that this is required for
duplication in the next cell cycle.
A confluence of results from several systems
has identified Polo-like kinase 4 (Plk4) as the
likely trigger for centriole formation in animal
cells13. Monica Bettencourt-Dias and Stefan
Duensing both showed that the levels of Plk4
are controlled by proteolysis in Drosophila and
mammalian cells, respectively. In both cell
types, depletion of components of the SCF (for
Skp1–Cullin–Fbox) ubiquitin-ligase complex
results in more Plk4 and formation of extra centrioles. Interestingly, Michel Bornens reported
centrosomal accumulation of Plk4 during mitosis and described a Plk4 autophosphorylation
event that stimulates degradation of the protein;
however, this seems to be independent of SCFmediated degradation. It is still not known how
Plk4 carries out its centriole-initiating magic,
but it seems likely that its level, localization and
activity are tightly controlled.
An early event in new centriole growth is
formation of the cartwheel, a nine-fold symmetrical structure at the base of the centriole.
Masafumi Hirono discussed his findings on
mutants of bld12 and bld10, the Chlamydomonas
orthologues of SAS-6 and Cep135; these two
proteins seem to be essential components of the
cartwheel, probably defining its nine-fold symmetry14,15. SAS-6 is at the centre of the cartwheel,
and loss of SAS-6 results in centrioles with nonnine numbers of triplet microtubules. Pierre
Gönczy reported that SAS-6 is phosphorylated
by ZYG-1 in C. elegans. ZYG-1 is a kinase related
to Plk4, and SAS-6 mutants that mimic phosphorylation at the ZYG-1 site can bypass the
requirement for the kinase, supporting the idea
that SAS-6 is the key target of ZYG-1 for centrosome duplication and is a central point of regulation of centriole assembly. Kevin O’Connell
showed that a conserved RNA-binding protein,
SZY-20 (known as PM20 in humans), acts antagonistically to ZYG-1 and regulates centrosome
size16, although the nature of the connection to
the RNA world is not clear.
Another conserved component originally
identified in C. elegans is SAS-4, thought to be
required to add centriolar microtubules to the
base structure17. Susan Dutcher’s genetic and
electron microscopic analysis of basal body
duplication in Chlamydomonas indicates that
spokes emanating from the central hub first
form an amorphous pinwheel before the cartwheel appears, perhaps as SAS-4 is recruited18.
Tang Tang presented evidence that levels of the
human orthologue CPAP are tightly regulated
during the cell cycle by proteolysis mediated by
the anaphase-promoting complex (APC). Tang,
Gönczy and Erich Nigg all noted that overexpression of CPAP results in growth of microtubule extensions from the end of the centriole,
extending its length. Alex Dammermann identified a protein, HYLS-1, that interacts with SAS-4
and found that it is required for cilium, but not
centriole, formation. In humans with hydroethalus syndrome, a mutation in HYLS-1 impairs
cilia assembly, adding this disease to the known
ciliopathies.
When the analysis of centriole and basal body
assembly from several different organisms is
combined, a picture of the stepwise centrosome
assembly pathway begins to emerge (Fig. 1).
Centrosomes and cell cycle signaling
Centrosome function is intimately tied to cellcycle progression, with characteristic changes
occurring in each phase of the cycle. Conserved
cell-cycle regulatory kinases, such as the cyclindependent kinases (Cdks), polo-like kinases
(Plks) and Mps1, control the function and
duplication of SPBs in fungi, and centrosomes
in metazoan cells. As a start to developing a more
complete understanding of the role of phosphorylation at the centrosome, Mark Winey described
an ambitious proteomics approach to examine
phosphorylation of all of the core components
of the S. cerevisiase SPB, whereas Harold Fisk
focused on Mps1 phosphorylation of centrin as
a control point for centriole duplication.
At the entry to mitosis in animal cells, more
PCM is recruited to centrosomes, and this
recruitment requires the activity of Plk1 and
Aurora A. Isabelle Vernos described experiments
in frog egg extracts to define the role of Aurora A
kinase activity at the centrosome19. Jens Lüders
talked about the connection between Plk1 and
γ−tubulin recruitment through the attachment
factor GCP-WD/Nedd1. Bringing some of these
threads together, Erich Nigg described recent
results from his lab, showing that Plk1 and Bora
cooperate to regulate the centrosomal levels of
Aurora A during mitotic entry in cultured cells20.
This is at least conceptually similar to the situation
in Schizosaccharomyces pombe, described by Iain
Hagan, in which recruitment of the polo kinase
Plo1 to the SPB is important for mitotic entry.
The results discussed above support the view
of the centrosome as a crucial signal transduction hub. This is perhaps most clearly true in
S. cerevisiase, where a surveillance system
known as the spindle-positioning checkpoint
(SPOC) monitors alignment of the mitotic
spindle at the bud neck and delays cell-cycle
progression until correct spindle orientation is
achieved. The target of the SPOC is the mitotic
exit network (MEN), and the ultimate target of
the MEN pathway is the Cdc14 phosphatase,
which antagonizes Cdk1–cyclinB activity. The
SPB serves as a scaffold for regulatory proteins
and a sensor for spindle alignment. Simonetta
Piatti’s analysis of the E3 ubiquitin ligases Dma1
and Dma2 suggested a new mechanism controlling the SPOC, whereas Gislene Pereira focused
on the dynamic association of Bub2 and Bfa1
with the SPB in cells with mis-aligned spindles
and how this is controlled by phosphorylation21.
Elmar Schiebel also discussed the important
role that phosphorylation has in this pathway, through analysis of Cdk1 regulation of
MEN components. Kathy Gould reported that
phosphorylation of Clp1, the Cdc14 ortholog
in S. pombe, promotes binding to Rad24 and
cytoplasmic retention during anaphase22. At
least some MEN proteins have orthologues in
higher eukaryotes, so an important future direction will be to elucidate their role in cell-cycle
progression. Also, the intimate association of
SPBs and centrosomes with the nuclear envelope seems to be important for regulating centrosome function and duplication, as discussed
by Sue Jaspersen on the basis of their studies on
the evolutionarily conserved SUN proteins.
Cell divisions: some more equal than
others
Several recent studies have highlighted the role
that spindle alignment and centrosome distribution play during developmentally important
asymmetric cell divisions, including in adult
stem cells, the germ line and the immune system. Yukiko Yamashita examined why adult
stem cells lose their ability to divide with increasing age during spermatogenesis in Drosophila.
These cells usually divide with the older centrosome anchored near the stem-cell niche, but she
nature cell biology volume 10 | number 12 | DECEMBER 2008
© 2008 Macmillan Publishers Limited. All rights reserved.
1377
mee t i n g r e p o r t
found that the number of male germ stem cells
with mis-oriented centrosomes increases with
time23. These cells also transiently arrested in the
cell cycle, explaining their decreased ability to
proliferate, and perhaps reflecting a checkpoint
similar to that described above for yeast.
Correct positioning of the mitotic spindle and
centrosomes is important to maintain cellular
identity, and defects in this process can result in
uncontrolled cell division. This was described
and discussed by Cayetano Gonzalez and Jordan
Raff, who used a transplantation system to study
tumorigenesis in flies. It has often been observed
that cancer cells have extra centrosomes, and
sometimes undergo multipolar divisions, which
might lead to some of the genetic instability
observed in such cells. Indeed, this is one of
the touchstones of the centrosome field, first
proposed by Theodor Boveri 100 years ago24.
However, the results from Gonzalez and Raff
are most consistent with centrosome abnormalities resulting in defects in asymmetric cell
division and thus resulting in over-proliferation
of stem cells25,26. Steve Doxsey presented results
suggesting that similar mechanisms might be
at work in vertebrates. He found that interfering with the function of centrosome proteins
in zebrafish caused phenotypes similar to those
described for ciliary proteins. Further analysis
in mammalian cells indicated that interfering
with IFT88, an intraflagellar transport protein,
resulted in misoriented spindles. This led to the
hypothesis that some of the phenotypes in ciliopathies might be due to defective cell division
plane orientation.
Cytokinesis failure is often cited as a mechanism responsible for generating the many cells
with extra centrosomes observed in tumours.
However, Kip Sluder presented evidence suggesting that cytokinesis failure is unlikely to be
the culprit in this case. In a heroic effort of timelapse imaging, the Sluder lab treated cultured
mammalian cells with cytochalasin to induce
cytokinesis failure, then observed cells over the
course of several cell cycles. Although they could
frequently recover tetraploid cells, most cells did
not contain extra centrosomes, and the tetraploid cells did not proliferate. This suggests that
centrosome amplification in tumour cells must
involve other steps, or perhaps multiple rounds
of cleavage failure. Susana Godinho’s analysis of
centrosome clustering in Drosophila S2 cells and
mammalian cancer cell lines indicates that even
when extra centrosomes are present, cells cope by
clustering centrosomes into poles of functional
bipolar spindle27. Remarkably, her results suggest
1378
that targeting centrosome clustering mechanisms
might be a way to specifically kill cancer cells.
Coming full circle
It is particularly satisfying to see a result that
clearly answers a long-standing question.
David Agard’s presentation on the structure of
the γ-tubulin complex from yeast did just that,
providing a molecular understanding of nucleation, the process that led most investigators to
the centrosome in the first place. Microtubule
nucleation at the centrosome involves γ-tubulin
and associated proteins. The γ-tubulin complex
purified from animal cells is ring-shaped, with a
size and diameter that suggest that it nucleates a
microtubule by directly templating it. A vexing
problem has been that yeast cells lack some of the
γ-tubulin complex associated proteins and also
lack a soluble ring complex. Combining purified
yeast proteins, from a collaboration with Trisha
Davis’ lab, and electron microscopy, Agard
showed that the simple combination of γ-tubulin,
its two closest binding partners Spc97 and Spc98,
and the linker protein Spc110 will form a ring
in vitro, with appropriate dimensions for microtubule nucleation. It is fitting, perhaps, that a
detailed understanding of γ-tubulin comes from
a study of the yeast proteins, given that γ−tubulin
was originally identified by Berl Oakley and colleagues in a genetic screen in Aspergillus28.
In conclusion, our understanding of these
complex organelles, which both control microtubule nucleation, and serve as important hubs for
cell signaling, has increased dramatically since
the first centrosome and SPB conference twelve
years ago. What was then a relative cell biological
backwater has now become what Tony Hyman
called “perhaps the most advanced organelle
with respect to combining genomics, proteomics
and cell biology”. No longer an enigma, the centrosome is now at the center of some of the most
important issues in biology, with the attendant
burning questions about its structure, function,
and duplication. Searching for the answers to
these questions will keep us busy until we meet
again in 2011 in Barcelona.
1. Hagan, I. M. & Palazzo, R. E. Warming up at the poles.
EMBO Rep. 7, 364–371 (2006).
2. Palazzo, R. Centrosome and spindle pole body dynamics. Cell Motil. Cytoskeleton 54, 148–194 (2003).
3. Stearns, T. & Winey, M. The cell center at 100. Cell 91,
303–309 (1997).
4. Wigge, P. A. et al. Analysis of the Saccharomyces
spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol. 141,
967–977 (1998).
5. Andersen, J. S. et al. Proteomic characterization of
the human centrosome by protein correlation profiling.
Nature 426, 570–574 (2003).
6. Kittler, R. et al. Genome-scale RNAi profiling of cell
division in human tissue culture cells. Nature Cell Biol.
9, 1401–1412 (2007).
7. Poser, I. et al. BAC TransgeneOmics: a high-throughput
method for exploration of protein function in mammals.
Nature Methods 5, 409–415 (2008).
8. Goshima, G. et al. Genes required for mitotic spindle
assembly in Drosophila S2 cells. Science 316, 417–
421 (2007).
9. Dobbelaere, J. et al. A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation
in Drosophila. PLoS Biol 6, e224 (2008).
10.Kilburn, C. L. et al. New Tetrahymena basal body protein components identify basal body domain structure.
J. Cell Biol. 178, 905–912 (2007).
11.Keller, L. C., Romijn, E. P., Zamora, I., Yates, J. R.,
III & Marshall, W. F. Proteomic analysis of isolated
chlamydomonas centrioles reveals orthologs of ciliarydisease genes. Curr. Biol. 15, 1090–1098 (2005).
12.Uetake, Y. et al. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed
human cells. J. Cell Biol.176, 173–182 (2007).
13.Nigg, E. A. Centrosome duplication: of rules and
licenses. Trends Cell Biol. 17, 215–221 (2007).
14.Hiraki, M., Nakazawa, Y., Kamiya, R. & Hirono, M.
Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr. Biol.
17, 1778–1783 (2007).
15.Nakazawa, Y., Hiraki, M., Kamiya, R. & Hirono, M. SAS-6
is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 17, 2169–2174 (2007).
16.Song, M., Aravind, L., Muller-Reichert, T. & O’Connell,
K. The conserved protein SZY-20 opposes the Plk-4
related kinase ZYG-1 to limit centrosome size. Dev.
Cell, advance online publication ,10.1016/j.devcel.2008.09.018 (2008).
17.Pelletier, L., O’Toole, E., Schwager, A., Hyman, A. A. &
Muller-Reichert, T. Centriole assembly in Caenorhabditis
elegans. Nature 444, 619–623 (2006).
18.Dutcher, S. K. Finding treasures in frozen cells: new
centriole intermediates. Bioessays 29, 630–4 (2007).
19.Sardon, T., Peset, I., Petrova, B. & Vernos, I. Dissecting
the role of Aurora A during spindle assembly. EMBO J.
27, 2567–79 (2008).
20.Chan, E. H., Santamaria, A., Sillje, H. H. & Nigg, E. A.
Plk1 regulates mitotic Aurora A function through βTrCPdependent degradation of hBora. Chromosoma 117,
457–69 (2008).
21.Caydasi, A. & Pereira, G. Spindle alignment regulates
the dynamic association of checkpoint proteins with
yeast spindle pole bodies. Dev. Cell, advance online
publication, 10.1016/j.devcel.2008.10.013 (2008).
22.Chen, C.-T. et al. The SIN kinase Sid2 regulates cytoplasmic retention of the Cdc14-like phosphatase Clp1
in S. pombe. Curr. Biol. 18, 1594–1599 (2008).
23.Cheng, J. et al. Centrosome misorientation reduces
stem cell division during ageing. Nature advance online
publication, doi: 10.1038/nature07386 (2008).
24.Bignold, L. P., Coghlan, B. L. & Jersmann, H. P.
Hansemann, Boveri, chromosomes and the gametogenesis-related theories of tumours. Cell Biol. Int.
30, 640–644 (2006).
25.Basto, R. et al. Centrosome amplification can initiate
tumorigenesis in flies. Cell 133, 1032–1042 (2008).
26.Castellanos, E., Dominguez, P. & Gonzalez, C.
Centrosome dysfunction in Drosophila neural stem cells
causes tumors that are not due to genome instability.
Curr. Biol. 18, 1209–1214 (2008).
27.Kwon, M. et al. Mechanisms to suppress multipolar
divisions in cancer cells with extra centrosomes. Genes
Dev. 22, 2189–203 (2008).
28.Oakley, C. E. & Oakley, B. R. Identification of γ-tubulin,
a new member of the tubulin superfamily encoded by
mipA gene of Aspergillus nidulans. Nature 338, 662–
664 (1989).
29.Strnad, P. & Gonczy, P. Mechanisms of procentriole
formation. Trends Cell Biol. 18, 389–396 (2008).
30.Bettencourt-Dias, M. & Glover, D. M. Centrosome biogenesis and function: centrosomics brings new understanding. Nature Rev. Mol. Cell Biol. 8, 451–463 (2007).
31.Azimzadeh, J. & Bornens, M. Structure and duplication of the centrosome. J. Cell Sci. 120, 2139–2142
(2007).
nature cell biology volume 10 | number 12 | DECEMBER 2008
© 2008 Macmillan Publishers Limited. All rights reserved.