Chemistry Notes
advertisement

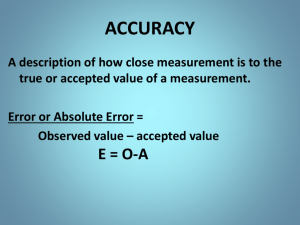
Chemistry Notes Unit 2 Measurement Metrics Scientific Notation Uncertainty Metric Measurement Fundamental – deals with single measurements that describes a single phenomenon Ex: time, distance, mass Derived – deals with two or more measurements that describe a single phenomenon Ex: m/s, Kg*m/s2, m/s2, N*m SOME REVIEW FOR YOU The metric system is much easier than our standard system of measurement because units with in the metric system are powers of ten. Ex: convert 1200 g to kg Answer 1.20 kg (just move the decimal) Ex: convert 40 oz. lbs Answer 2 ½ lbs (have to do some math) SOME REVIEW FOR YOU Metric Measurement Some basic units see table 2.1 on page 29 Length meters Time seconds Mass kilogram Metric Prefixes see table 2.2 on page 29 MicroMillCentiDeci Kilo- 0.000 001 m 0.001 m 0.01 m 0.1 m 1000 m SOME REVIEW FOR YOU Converting between metric units Remember SSL (Smaller units to larger units move decimal to the left) K,H,D,b,d,c,m E.g. When you convert from milli- to deci- you move the decimal point two places SOME REVIEW FOR YOU Conversion with Unit Analysis Method: Used to convert standard to metric Standard form below: ## given units ## desired units = desired units ## given units If set up correctly, the given units will cancel out. That how you know you set it up right. SOME REVIEW FOR YOU Mass = the amount of matter in an object Measured in kilograms (kg) Not to be confused weight. Weight is more comparable to force, W = mg A balance is an instrument used to determine mass SOME REVIEW FOR YOU Mass v. Weight REVIEW Length = the distance covered by a straight line segment between two points Measured in meters (m) Time – interval between two occurrences Measured in seconds (s) SOME REVIEW FOR YOU Temperature = the average kinetic energy of the particles that make up matter. Measured in Kelvin (K) Kelvin is a scale based on molecular motion. We also use Celsius (oC) To convert from (oC) to K: K = 273.15 + (oC) (oC) = K – 273.15 SOME REVIEW FOR YOU Example problem: convert 25oC to Kelvin 298 K Example problem: convert 400 Kelvin to oC 127 oC SOME REVIEW FOR YOU Scientific Notation Used in science because scientist often work with very large or very small numbers Ex: Astronomers, Bacteriologists, Chemists SOME REVIEW FOR YOU To add and subtract numbers that are in scientific notation you have to: Be sure numbers are in the same units NOT one in grams and one in kilograms Be sure the numbers are to the same power Examples: 2.30 X 1016 + 3.44 X 1014 = __________ 1.14 X 107 – 3.11 X 109 = __________ SOME REVIEW FOR YOU To multiply or divide numbers that are in scientific notation you have to: Add exponents if you are multiplying Subtract exponents if you are dividing Examples (6.87 X 1087) (8.24 X 1073) = _________ (4.87 X 1036) / (8.55 X 1021) = ________ HOMEWORK GCWS 2A (More) Uncertainty Recall: in chemistry (or any science) we deal with a lot of uncertainty. Question: how else do we handle uncertainty? Answer: Calculations like percent error and significant digits allow scientist to see how far off they may be. Uncertainty Percent Error: is calculated as seen below Percent Error = Experimental value – Theoretical value X 100 Theoretical value Because absolute value is used % error will always be a positive number. Uncertainty Ex: what is the % error of an experiment where a student produces 43 g of sodium sulfite from the reaction including sodium, sulfur and oxygen? The theoretical mass of sodium sulfite was 49.2 g. 12.6 % Ex: what is the % error of an experiment where a student observed that the time a certain reaction lasted was 15.24 s? The theoretical time that was calculated was 15.01 s. 1.53% MORE HOMEWORK DO GCWS 2C Uncertainty What is the measurement below? 11.65 cm Uncertainty All measurements are approximates. Sig figs allow us to be honest about how precise are measurements are Example Postage Scale: 3 g - precise to 1 significant figure Two-pan balance: 2.53 - precise to 3 significant figures Analytical balance: 2.531 - precise to g 4 significant figures As we improve the sensitivity of the equipment used to make a measurement, the number of significant figures increases. Uncertainty Significant Figures Question: Which of the following measurements is the most precise? 12.000 m 12 m Uncertainty Answer: 12.000 m is more precise because what is being said is that this measurement was to the closest 0.001 m opposed to being measured to the closest meter. These numbers are not saying the same thing. 12.000 is saying the measurement is between 11.999 m and 12.001m 12 is saying the measurement is between 11.5 m and 12.5 m 12.000 contains 5 significant digits whereas 12 only contains 2 All numbers but the last ones are certain. Uncertainty Determining Significant digits: All non-zero numbers are significant 95.32 4 sig figs Zeros after decimal points are significant 54.100 5 sig figs Zeros between non-zero numbers are significant 120.0000001 10 sig figs Zeros that are place holders are not significant 0.000045 2 sig figs 0.001000 4 sig figs Uncertainty Adding and subtracting significant figures Answer should have as many decimal places as the measured number with the smallest number of decimal places. EX: Uncertainty Multiplying and Dividing with significant figures answer should have as many significant figures as the measured number with the smallest number of significant figures. Ex: Summary What questions do you still have? What was unclear?