RCC Lab 4 S14
advertisement

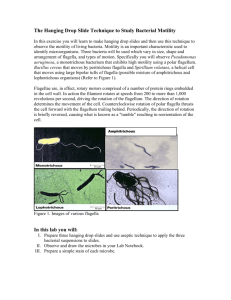
Lab #4 Review of Lab #3 • Oxygen requirements • Obligate aerobes (B. subtilis) • Obligate anaerobes (C. sporogenes) • Facultative anaerobes (E. coli, K. pneumoniae, and S. epidermidis) • Microaerophilic • Slant – only aerobic and facultative anaerobic • Motility tall – only aerobic (top) and facultative anaerobic (throughout) • Thioglycolate broth – all organisms will grow but in a different location in the broth Review of Lab #3 • Oxygen requirements Review of Lab #3 • Catalase test • Organisms that utilize oxygen produce enzymes to help protect against toxic oxygen derivatives (H2O2, O2-, etc.) • Catalase enzyme H2O2 H20 + ½ O2 (Production of O2 is seen as bubbles) • E. coli • B. subtilis • K. pneumoniae • S. epidermidis Catalase Positive Review of Lab #3 • Endospore staining • Positive result = spores are present (you see green spores and pink vegetative cells) • Negative result = no spores are present (you only see pink vegetative cells) • Vegetative cells will be present in both cases! • B. cereus – spore positive • B. subtilis – spore positive • E. coli – spore negative • K. pneumoniae – spore negative • S. epidermidis – spore negative Lab #4 – Streak plate method (4D) • Culture technique used to obtain isolated colonies obtain a pure culture Lab #4 – Streak plate method (4D) • Streak plate method (pg. 26 – 29): • Sample is only take once – only for quadrant 1 • Streak from top to bottom – don’t go up and down • Flame loop in between each quadrant!! • Work aseptically!! Lab #4 – Streak plate method • Each student streak 3 plates – lots of practice! • Label plates properly • Name • Date • Organism • Incubate the plates INVERTED • Why? Lab #4 – Acid fast staining (9) • Type of differential stain • Used to identify members of the genus Mycobacteria • Mycobacterium tuberculosis • Mycobacterium leprae • Mycobacteria cell walls have a high concentration of waxy lipid substances (mycolic acid) • Prevent dyes from staining the cells • Heat is used to help stain cells • Primary stain: Carbolfushin • Counterstain: Methylene blue Lab #4 – Acid fast staining • Method (Pg. 45-46): • Prepare smear • Add 2 loopfuls of 1% albumin (helps hold bacteria onto slide) • Mix in bacteria aseptically • Air dry and heat fix • Flood slide with Carbolfushin • Blow torch for 5 minutes, intermittently DON’T LET STAIN • • • • • • DRY Rinse with water Decolorize with Acid Alcohol until color stops to run Rinse with water Cover smear with Methylene blue for 1 minute Rinse with water Blot dry and observe under microscope Lab #4 – Acid fast staining • Acid fast positive = Red cells • Acid fast negative = Blue cells • Only Mycobacterium species will be Acid fast positive resist de-colorization and retain primary stain (Carbolfushin) Lab #4 – Acid fast staining • Each student prepare two stains: • M. tuberculosis • S. epidermidis • Record results on pg. 47 Lab #4 – Hanging drop method (8A) • Method to determine motility (pg. 41 - 42) • Use a broth culture • Need a depression slide, coverslip, and Vaseline • Smear Vaseline on palm scrape onto the four edges of • • • • coverslip (don’t mix vaseline with culture!) Transfer loopful of bacterial culture onto coverslip Place depression slide, concave side down, onto coverslip DON’T PRESS CAREFULLY invert slide see the hanging drop Observe under microscope for motility Lab #4 – Hanging drop method • Microscope observation: • Start at 10X objective look for the edge of the drop • Center the edge of drop in field of view • Switch to 40X objective FINE focus • Bacteria will appear “ghost like” within the drop (no stain!) • Observe to distinguish motility vs. Brownian motion • Motility is distinct movement from point A to point B • Brownian motion = jiggling in one spot/ gliding in direction Lab #4 – Hanging drop method • Each student prepare a hanging drop for: • S. epidermidis • E. coli • Record motile or non-motile on pg. 44