Unit 10 - Gases - Davis
advertisement

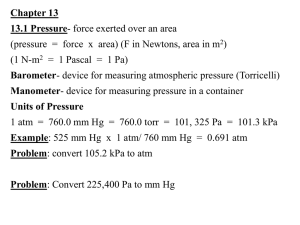
Unit 10 - Gases Kinetic Molecular Theory • All matter is composed of tiny particles that are in constant motion • Gases are point masses that: – Are in constant motion – Have elastic collisions – Have no attractive forces between particles http://phet.colorado.edu/new/simulations /sims.php?sim=Gas_Properties 14.1 The Gas Laws Defining Gas Pressure • According to the kinetic theory, all matter is composed of particles in constant motion, and pressure is caused by the force of gas particles striking the walls of their container. – The more often gas particles collide with the walls of their container, the greater the pressure. – Therefore the pressure is directly proportional to the number of particles. Physical Properties • Temperature (average kinetic energy) – ↑ temperature, ↑ velocity – Units – always Kelvin (add 273 to Celsius temp.) – Standard Temperature – 0°C (273 K) – Absolute Zero – All motion stops • 0 K or -273°C Physical Properties • Volume (space taken up by movement of molecules) – Gas will always occupy total volume of container • Transfer gas from a 2 L to a 1 L bottle & its volume changes from 2 L to 1 L – Gases can expand or be compressed • Ex. 1 mol dry ice = 28 cm3 1 mol CO2 gas = 25000 cm3 Physical Properties • Volume cont. – To expand or compress gas must have: • Container capable of changing volume (ex. balloon) • ↑ T = more volume • ↑ P = ↓ V (squeezes) – Gases diffuse from high to low concentrations Physical Properties • Pressure – Force over a given area exerted by moving gas molecules – Depends upon number & force of collisions • Amount of gas (moles) • Volume of gas • Temperature Physical Properties • Pressure cont. – Units: • Pascals (N/m2) • Mm Hg • Torrs • Atmospheres • PSI (pounds per square inch) – Standard Pressure: 1 atm = 101.3 kPa = 760 mm Hg = 760 torrs Physical Properties • Barometer – An instrument that measures atmospheric pressure (the pressure exerted by the atmosphere). – The height of the mercury column measures the pressure exerted by the atmosphere. • Manometer – measures pressure of other gases compared to atmospheric pressure 14.1 The Gas Laws Defining Gas Pressure • The pressure of a gas is the force per unit area that the particles in the gas exert on the walls of their container. – As you would expect, more air particles inside the ball mean more mass inside. • Note: the pressure of a gas is directly proportional to its mass. Physical Properties • Pressure cont. – You can change pressure by: • Adding heat – molecules move faster; more collisions; more pressure • Increase volume – same # atoms; ↑ space; ↓ pressure – STP = Standard Temperature & Pressure (0°C & 1 atm) Physical Properties • Atmospheric pressure is the result of collisions of air molecules with objects. • Atmospheric pressure decreases with an increase in altitude. The air around the earth “thins out” at higher elevations. Physical Properties Pressure decreases with increasing altitude Graham’s Law • Diffusion – gas molecules move from high concentration to low concentration • Effusion – gas molecules pass through a small hole • At the same temperature heavier molecules are slower, lighter molecules are faster 14.1 The Gas Laws The Gas Laws The gas laws apply to ideal gases, which are described by the kinetic theory in the following five statements. – Gas particles do not attract or repel each other. – Gas particles are much smaller than the spaces between them. – Gas particles are in constant, random motion. – No kinetic energy is lost when gas particles collide with each other or with the walls of their container. – All gases have the same kinetic energy at a given temperature. Gas Laws • Relate: Volume – Temperature – Pressure • Boyle’s Law – Effect of pressure on volume – ↑P ↓V (inverse relationship) – Temp. is constant – If you double P; V will be halved – a) If the pressure of a gas increases, its volume decreases proportionately. – b) If the pressure of a gas decreases, its volume increases proportionately. – P1V1 =P2V2 Gas Laws • Charles’ Law – Effect of temperature on volume (the volume of the gas increases in proportion to the increase in Kelvin temperature). – – – – – ↑T ↑ V (direct relationship) T must be in Kelvin Pressure is constant If you double T, V will double V1 = V2 T1 T2 Gas Laws • Gay-Lussac’s Law – Effect of temperature on pressure – ↑T ↑P (direct relationship) – T must be in Kelvin – Double T, P will also double – Half T, P will also be one-half – P1 = P2 T1 T2 Gas Laws • Combined Gas Law P1V 1 P 2V 2 T1 T2 • If you do not have a change in one of the variables replace it with a one or omit it! • Only need ONE FORMULA!!!!!! Practice Problems • Write down all givens; Change °C to K; Think about relationship; Finally solve problem A balloon inflated in an air conditioned room at 27oC has a volume of 2.0 L. If it is heated to 57oC and the pressure remains constant, what is the new volume? T1= 27°C + 273= 300K V1= 2.0 L P1= constant V1 T1 V2 T2 2.0 L 300K ?L 330K T2= 57°C + 273= 330K V2 = ? L P2= constant ?L ( 330K )( 2.0 L ) 300K 2.2 L Practice Problems 1. A gas in an aerosol can is at a pressure of 1 atm and 27°C. If the can is thrown into a fire, what is the internal pressure of the gas if the temperature reaches 927°C? 2. A 25 mL balloon at 1.25 atm and 45°C is released and rises up to an atmospheric pressure of 0.816 atm where the volume of the balloon changes to 100 mL. What temperature is the gas at the new pressure? Real vs. Ideal Gases Real Ideal Made up of particles Made up of particles Particles in constant motion above 0 K Particles in constant motion above 0 K When particles collide, one loses energy & one gains energy Particles have elastic collisions Particles attract each other Particles have no attraction for each other Particles (atoms, etc.) actually take up some space Particles occupy no space. Volume of gas is volume of container Ideal Gases • Ideal gases don’t really exist, but many gases do act ideally under certain conditions • When gas molecules are far apart and not able to attract one another, they act ideally • This usually occurs at High Temperatures and Low Pressure 14.3 The Ideal Gas Law The Ideal Gas Law • The pressure, volume, temperature, and number of moles of gas can be related in a simpler, more convenient way by using the ideal gas law. – The following is the law’s mathematical expression, PV = nRT where n represents the number of moles. – The ideal gas constant, R, already contains the molar volume of a gas at STP along with the standard temperature and pressure conditions. Ideal Gas Law • Solving Problems – Write down the givens in the problem – Make sure all the units are in L, mol, atm, and K – Plug into the equation (PV=nRT) and make sure unwanted units cancel Ideal Gas Law 2.0 mol oxygen gas are placed in a 10 L container at 20°C. What is the pressure? n=2.0 mol V=10 L T=293K R= 0.0821 L atm/mol K P=? PV=nRT P=nRT/V P=[(2.0mol)(0.0821 L atm/mol K)(293K)]/(10 L) P=4.8 atm Ideal Gas Law: PV=nRT • Relates P, V, T and Moles (n) • For any ideal gas VP/nT is constant; we call this contant R • We can calculate R by inserting the values for STP and the molar volume of a gas at STP… so that… R VP nT ( 22.4 L )(1atm) (1mol )( 273K ) 0.0821 L atm mol K • Watch units when using Ideal Gas Law!!!! Practice Problems 1. How many moles of H2 must be put into a 200 mL container at 25°C to get 1.5 atm? 2. 0.8 g of gas occupies a volume of 372 mL at 100°C and 800 torr. Find its molar mass. 3. Find the density of NO2 at 100°C and 800 torr Dalton’s Law of Partial Pressure • If several gases are mixed together – They behave as if they were each alone in the container – Each gas exerts its own pressure – So Dalton said: Total Pressure = sum of the partial pressures of each gas PT=P1+P2+P3+… Dalton’s Law of Partial Pressure • Examples: • Gas A exerts a pressure of 1.5 atm in a 2 L container. Gas B exerts a pressure of 2.0 atm in the same container. What is the total pressure? • Gas A & B (above) are in a 1 L container. What is the total pressure Dalton’s Law of Partial Pressure • 2 mol O2, 3 mol N2, and 5 mol CO2 are in a 3 L container at 1000 torr. What is the volume of each gas? (mol gas/total mol) x total pressure O2 = (2/10) x 1000 = 200 torr N2 = (3/10) x 1000 = 300 torr CO2 = (5/10) x 1000 = 500 torr Gas Density • Density = Mass/Volume (units=g/L) • At STP, Gas density = Molar mass/22.4L – Remember 1 mol=22.4L, Molar Mass=g/mole • If two gases have the same T, P, V; they will have the same number of particles or moles! • 22.4L can be use to change mol & volume ONLY AT STP • Example: 11.2 L H2 1 mol H2 22.4 L H2 Gas Density • Gas density changes with P & T – If T or P changes does mass change? Does volume change? – If volume increases, density will ________? • To predict density changes, determine what volume will do. Density will do the opposite. Gas Density • To find the new density – Find the new volume using Combined Gas Law – Divide same mass by new volume T 1D1 T 2 D 2 P1 P2 Touch Down Pass 1 = Touch Down Pass 2 Gas Density The density of a gas is 1.5 g/L at 20°C and 700 torr. Find the density at STP. P1 = 700 torr P2 = 760 torr V1 = 1L V2 = ? L T1 = 293K T2 = 273K ( 700torr)(1L ) 293K ( 760torr)(V 2 ) 273K V 2 0.86 L New Density: mass = 1.5g = 1.74 g/L V2 0.86L Practice Problems 1. The density of O2 is 1.43 g/L at STP. Find the density at 50°C. 2. What is the density of CO2 at STP? 3. What is the density of CO2 at 50°C and 780 mmHg? Stoichiometry Review • Remember that the coefficients stand for moles, molecules, and Liters @STP • Review the steps • Balance the equation • Write want and given • Convert given to moles • Multiply by ratio want / given • Convert to what you want • Adjust from STP if required 14.4 Gas Stoichiometry Gas Stoichiometry • Now that you know how to relate volumes, masses, and moles for a gas, you can do stoichiometric calculations for reactions involving gases. • Ammonium sulfate can be prepared by a reaction between ammonia gas and sulfuric acid as follows. • What volume of NH3 gas, measured at 78°C and a pressure of 1.66 atm, will be needed to produce 5.00 x 103 g of (NH4)2SO4? 14.4 Gas Stoichiometry Gas Stoichiometry Using Mass • Finally, use the ideal gas law equation to calculate the volume of 75.68 mol NH3 under the stated conditions. – Solve the equation for V, the volume to be calculated. – Convert the temperature to kelvins, substitute known quantities into the equation, and compute the volume. • Notice that the values for the molar mass of (NH4)2SO4 and the number of moles of NH3 have more than three significant figures, whereas the calculated volume has only three. 14.4 Gas Stoichiometry Gas Stoichiometry Using Mass • When you do a problem in a stepwise way, you should maintain at least one extra significant figure in the intermediate values you calculate. • Then, round off values only at the end of the problem.