Histamine and Antihistamines
advertisement

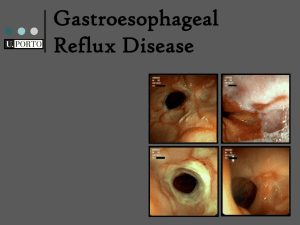
Gastric Acid Modifiers or Gastric Acid Suppression (GAS) November 27, 2007 Frank F. Vincenzi $13 billion market in 1998, inc. at 3%/yr Drug list for gastric acid modifiers • • • • • • • • • cimetidine (Tagamet® & generic, OTC) famotidine (Pepcid® & generic, OTC) lansoprazole (Prevacid®, ) misoprostol (Cytotec®) nizatidine (Axid®) omeprazole (Prilosec®) pirenzepine (not avail in U.S.) ranitidine (Zantac®) sucralfate (Carafate®) Trivial mechanisms of gastric acid modification • Acid neutralization and/or physical protection – Sucralfate (Carafate®) ( duodenal>gastric>>>GERD) (may decrease bioavailability of other drugs) – Aluminum hydroxide + magnesium hydroxide (Gelusil®, Maalox®, Mylanta®) – Bismuth subsalicylate (Pepto-Bismol®) & other bismuth compounds – Calcium carbonate (Tums®) Gastric acid secretion Factors affecting gastric acid secretion MC = mast cell like cell or enterochromaffin like cell Histamine receptors • H1 - smooth muscle, exocrine glands, vascular endothelium, brain; coupled to phospholipase C, leading to IP3 and diacylglycerol (DAG) • H2 - parietal cells, heart, vascular smooth muscle, mast cells, brain; coupled to cAMP production • H3 - presynaptic, brain, myenteric plexus (no therapeutic applications, yet) H2 antihistamine prototypes: decreased gastric acid secretion • cimetidine (Tagamet®) binds the androgen receptor inhibits CYP (2C19 ; 1A2, 2D6) • well absorbed, poor CNS penetration half life ~ 2 hours (800 mg HS, 300 mg QID, 400 mg AM & HS) • ranitidine (Zantac® half life ~ 2.5 hours (150 mg BID, 300 mg HS) • famotidine (Pepcid®) • nizatidine (Axid®) (better bioavailability) Histaminergic (H2) and muscarinic stimulation of gastric acid secretion in isolated parietal cells: antagonism by cimetidine and atropine, respectively Indication for H2 antihistamines: chronic recurrent ulcers • Ulcers induced by drugs (NSAIDs) (may use sucralfate and/or misoprostol) • Ulcers associated with hypersecretion (Zollinger-Ellison syndrome) • Ulcers associated with malignancy • Helicobacter pylori-positive ulcers (with inflammation of stomach & duodenum most common type of ulcer) Indication for H2 antihistamines: chronic recurrent ulcers • Ulcers induced by drugs (NSAIDs) • Ulcers associated with hypersecretion (Zollinger-Ellison syndrome) • Ulcers associated with malignancy • Helicobacter pylori-positive ulcers (with inflammation of stomach & duodenum most common type of ulcer) H2 antihistamines: adverse reactions • • • • Headache Dizziness Diarrhea, constipation Skin rashes • For cimetidine: loss of libido, gynecomastia, impotence drug interactions based on inhibition of CYP1A2, CYP2C19 and CYP2D6, less with ranitidine, no inhibition with famotidine or nizatidine A new way to decrease acid secretion: proton pump inhibitors (PPIs) • omeprazole (Prilosec®) [recently esomeprzole (Nexium®)] • lansoprazole (Prevacid®) • Inhibit the H/K pump of the parietal cell plasma membrane - probably from the outside - somewhat like digitalis inhibition of Na/K pump - but irreversibly • Adverse reactions: headache, nausea, diarrhea (and, rarely, Stevens-Johnson syndrome) Factors affecting gastric acid secretion MC = mast cell like cell or enterochromaffin like cell Omeprazole: irreversible inhibition of the proton pump enzyme Proton Pump Inhibitors (PPIs): Common Features • Enteric coating prevents premature destruction • Metabolized extensively by liver (2C19 & 3A4) - avoid hepatic disease • Typically a short plasma half-life, but a long duration of action - covalent inactivation of the proton pump • Absorbed and then ion-trapped and activated in acidic stomach. • Best taken with or before meals (acid labile) • Daily dosing may result in 70% of proton pumps inactivated in 2-5 days • Hypergastrinemia in 5-10% of patients - tumors? Omeprazole (Prilosec®): Indications • • • • Active duodenal ulcer Helicobacter eradication (with combotherapy) Severe erosive esophagitis Symptomatic poorly responsive gastroesophageal reflux disease (GERD) • Pathological hypersecretory conditions (ZollingerEllison syndrome Omeprazole & esomeprazole compared clinically in patients with Gastroesophageal Reflux Disease (GERD) 100 90 80 70 60 50 40 30 20 10 0 esomeprazole % of day at pH >4 omeprazole Adapted from Baker, 2001 Clinical Trials of Medical Treatment of Gastroesophageal Reflux Disease (GERD) Kahrilas, 1999 Treating GERD I Sporadic - lifestyle, weight, antacids or H2 block PRN II Frequent (2-3 x/wk) - PPIs, better than H2 blockers III Chronic unrelenting - PPIs 1-2 x/day Some concerns about long term GAS • Malabsorption of nutrients? • Increased susceptibility to enteric infection? • Development of GI neoplasia (from inc gastrin)? Adverse Reaction Concerns Malaz Boustani, et al., Journal of the American Geriatrics Society, August, 2007 A 5-year observational study included 1,558 cognitively normal African-Americans aged 65 and older. After controlling for other possible factors, nearly 18% of H2A users studied exhibited signs of cognitive impairment. "Taking these medications continuously appears to put older African-Americans at greater risk for the development of cognitive impairment," said "We need to study this further to determine how acid blockers might be causing or creating this effect and if it occurs only in African-Americans." Gastric acid suppression (GAS) by H2 receptor antagonists (H2RAs) or proton pump inhibitors (PPIs): association with pneumonia • • • • • > 360,000 pts., 5551 first pneumonia >10K H2RAs, >12K PPIs (some both)* 4.5 x (3.8-5.1) more often on GAS than not** ~1 case per 226 PPI pts (NNH) RR of first pneumonia 1.89 (1.36-2.62) for H2 blkrs, and 1.63 (1.07-2.48) for PPIs • Use of gastric acid-suppressive therapy was associated with an increased risk of communityacquired pneumonia. Laheji et al., JAMA 292: 1955-1960, 2004 Adverse Reaction Concerns Gulmez et al., Arch Intern Med. 2007;167:950-955. Use of PPIs within the past 7 days was associated with a fivefold higher risk of community acquired pneumonia and use of > 12 weeks was associated with increased risk of CAP (OR 1.3). Adverse Reaction Concerns Robertson DJ, Larsson H, Friis S, Pedersen L; Baron JA; Sorensen HT, Proton Pump Inhibitor Use and Risk of Colorectal Cancer: A PopulationBased, Case-Control Study Gastroenterology. 2007;133:755-760 Long-term proton pump inhibitor therapy at a regular dose is not associated with a significantly increased risk of colorectal cancer.