Adding Fermi-Dirac Statistics to the Drude Model = Sommmerfield
advertisement
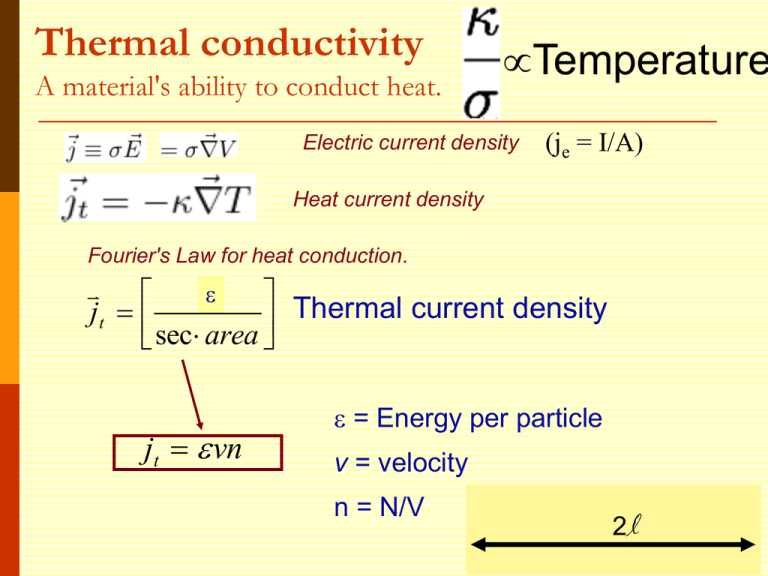
Thermal conductivity A material's ability to conduct heat. Temperature Electric current density (je = I/A) Heat current density Fourier's Law for heat conduction. E Thermal current density jt sec area jt vn = Energy per particle v = velocity n = N/V 2l Thermal conductivity Heat current density jt vn Heat Current Density jtot through the plane: jtot = jright - jleft Limit as l gets small: About half the particles are moving right, half to left. x Thermal conductivity x v v v Thermal conductivity (expanding to 3d) Heat current density x T T x v 2 vx 1 2 jt v cv T jt T 3 2 vy 2 vz 2 3 vx 2 1 2 v cv 3 How does / depend on temperature? Thermal conductivity 1/3 cvv2 = ne2/m 1/3 cvmv2 = ne2 Drude applied kinetic theory of gases ½ mv2 = 3/2 kBT = cvkBT ne2 The book jumps through claiming a value for cv Classical Theory of Heat Capacity When the solid is heated, the atoms vibrate around their sites like harmonic oscillators. The average energy for a 1D oscillator is ½ kT. Therefore, the average energy per atom, regarded as a 3D oscillator, is 3/2 kBT, and the total energy density is 3/2 nkBT where n is the conduction electron density and kB is Boltzmann constant. Differentiation w.r.t temperature gives heat capacity 3/2 n kB = 3/2 n kB2T ne2 = 3kB2T / 2e2 Thermal conductivity optimization To maximize thermal conductivity, there are several options: Provide as many conduction electrons as possible Make crystalline instead of amorphous irregular atomic positions in amorphous materials scatter phonons and diminish thermal conductivity Remove grain boundaries free electrons conduct heat more efficiently than phonons (=lattice vibrations). gb’s scatter electrons and phonons that carry heat Remove pores (air is a terrible conductor of heat) What happens? The Seebeck Effect A temperature gradient generates an electric field E = QT, where Q is known as the thermopower = -cv / 3ne Seebeck and the reverse (Peltier) Effects ~ millivolts/K for (Pb,Bi)Te The Seebeck effect is the conversion of temperature differences directly into electricity. Applications: Temperature measurement via thermocouples; thermoelectric power generators; thermoelectric refrigerators; recovering waste heat Demo: https://www.youtube.com/watch?v=bt5o_rn0FmU Many open questions: Why does the Drude model work relatively well when many of its assumptions seem so wrong? In particular, the electrons don’t seem to be scattered by each other. Why? Why is the actual heat capacity of metals much smaller than predicted? From Wikipedia: "The simple classical Drude model provides a very good explanation of DC and AC conductivity in metals, the Hall effect, and thermal conductivity (due to electrons) in metals. The model also explains the Wiedemann-Franz law of 1853. "However, the Drude model greatly overestimates the electronic heat capacities of metals. In reality, metals and insulators have roughly the same heat capacity at room temperature.“ It also does not explain the positive charge carriers from the Hall effect. Objectives By the end of this section you should be able to: Apply Sch. Equation to a metal Apply periodic boundary conditions Start to understand k space Determine the density of states and Fermi energy Find the Fermi temperature, velocity, etc. Improvement to the Drude Model Sommerfield recognized we needed to utilize Pauli’s exclusion principle Typically, this is the only difference Electrons cannot all be in the lowest energy state, since this would violate the Pauli Principle. Number of electrons per unit volume f(v) Fermi-Dirac Maxwell-Boltzmann = N/V Normalization condition solves for constants Another common way to write is f(E) Sommerfield still assumes the free electron approximation U(r) U(r) Neglect periodic potential & scattering Reasonable for “simple metals” (Alkali Li,Na,K,Cs,Rb) What does this remind you of? The Quantum Analogy These conduction electrons can be considered as moving independently in a square well and the edges of well corresponds to the edges of the sample. (ignores periodic potential from atoms) A metal with a shape of cube with edge length of L Cube V=L3 U 2 d 2 U ( x) 2 2m dx in 1D Inside U=0, for 3 dimensions: 0 L How do we go about solving this? U 0 L Possible Boundary conditions 1. Common: Ψ(0)=0 and Ψ(L)=0 Standing waves. Wells aren’t really infinite 2. Periodic: Ψ(x,y,z)= Ψ(x+L,y,z) Eigenstates 1 ikr (r ) e V Known as a running wave with eigenvalues 2k 2 2m To Compare, Let’s Remind Ourselves of the Standing Wave Solution U Boundary conditions Ψ(0)=0 and Ψ(L)=0 0 L Eigenstates where ( x) A sin( kx) with eigenvalues n k L 2k 2 E 2m n y nx n z , ky , kz Or in 3D: k x L L L Similar idea for running waves: e Where nx, ny and nz are integers ik y L ik x L ik z L 1 e How to find A? e Wavefunctions: Ideal Quantum Well 1D ( x) A sin( kx) (x) n k L standing waves 19 In your group, write the wavefunction for the lowest three energies. Semiconductor Quantum Well More about this diagram later today In CoFe Optical Detection of Spin hot Polarization in Quantum Wells e GaAs/InGaAs/GaAs external magnetic field CoFe e Alumina tunnel barrier This is a very simple spin-selective device. Electrons of one angular momentum are favored as they travel past the Schottky barrier due to the external magnetic field and spin filtering in the CoFe. They then fall into the quantum well and recombine with holes. Emission from the quantum well gives a good probe of spin. hn h GaAs: n InGaAs i i p The wave vector k is very important! Eigenstates 1 ikr (r ) e V with eigenvalues 2k 2 2m To see why, note that is an eigenstate of the momentum operator p k is the wave vector ik r e (Will explore more in Ch.5) Momentum space or k-space is the set of all wavevectors k, associated with particles - free and bound All points in a crystal that have an identical environment are described by one point in k space. This allows us to dramatically reduce the size of many atom systems. The Density of Levels (Closely related to the density of states) As we’ll see next time, we will often need to know the number of allowed levels in k space in some k-space volume If >>2/L, then the number of states is ~ / (2/L)3 (in 3d) Or V/ (2)3 Then the density of those levels is N/ or V/83 Summing over k space Since the volume of k space is V/83, summing any smooth function F(k) over k space can be approximated as: Will show an example later Consider a spherical reason Let’s find the number of allowed k values inside a spherical shell of k-space of radius kF 3 4k F 3 The number of allowed values of k 4k F V kF N ( )( 3 ) 2 V 3 8 6 3 3 Since there are two spin states for each k 3 N kF n 2 V 3 Warning: The Fermi level will be defined slightly differently for nonmetals. The Fermi Sphere The k-space sphere with radius kF is called the Fermi sphere. 3 n N kF 2 V 3 kz 2 kF F 2m 1/ 3 3 2 N kF V Fermi surface kF ky kx 2 3 N EF 2m V 2 2 2/3 If we convert k-space to energy space, the resulting radius of the energy sphere surface would give us the cutoff between occupied and unoccupied energy levels. The surface of the Fermi sphere represent the boundary between occupied and unoccupied k states at absolute zero for the free electron gas. Definition of the Work Function Additional energy above the Fermi level required to remove electrons from the solid EF fermi level =work function (3-4eV) 28 Fermi Energy in terms of the Bohr radius 2 a0 0.529 Å – Bohr radius 2 me 2 Bohr radius = mean radius of the orbit of an electron around the nucleus of a hydrogen atom at its ground state 2 2 kF e 2 F ( )( k F ao ) 2m 2 ao Recognize? Ground state energy of hydrogen atom 2 e 13.6eV rydberg ( Ry ) 2ao Electrons in 3D Infinite Potential Well Group: What is the ground state configuration of many electrons in the 3D infinite potential well? Consider the case of solid with 34 electrons. Determine the energy of each electron relative to 2h2 Eo 2mL2 . How many electrons are of each energy? Take the ground state to be when n1 = n2 = n3 = 1 Extra slides we may not have time to cover (just extra examples) Summing the energy density over k space Since the volume of k space is V/83, summing any smooth function F(k) over k space can be approximated as: An extra factor of 2 because of spin dk=k2sin dk d d Energy per Electron E/N in the ground state Combining: 3 N kF n 2 V 3 E V 2 2 E 3 hk 3 F N 10 m 5 Fermi Temperature F kBTF The density of copper is 8.96 gm/cm3, and its atomic weight is 63.5 gm/mole. (assume valence of 1) 3 2 N EF 2m V 2 (a) (b) 2/3 Calculate the Fermi energy for copper. Find the classical electron velocity from EF= ½ mvF2 and Fermi temperature. E k T F B F Pressure and Compressibility of an Electron Gas (Skip if time low, result most important, in book) 3 N EF 2m V 2 2 2/3 Pressure is defined as E/ V for constant N, so the pressure on an electron gas is Reminder: Effective Radius rs – another measure of electronic density = radius of a sphere whose volume is equal to the volume per electron (mean inter-electron 3 spacing) 2 4rs V 1 a0 0.529 Å – Bohr radius 2 me 3 N n in metals rs ~ 1 – 3 Å (1 Å= 10-8 cm) Combine with above: 1/ 3 3 N kF V 2 3 1/ 3 k F (3 ) 3 4rs 1.92 3.63 kF rs rs / ao 2 Å-1 rs/a0 ~ 2 – 6 9 1/ 3 ( ) 1.92 4 rs rs