Heat Capacity of Metals PreLab
advertisement
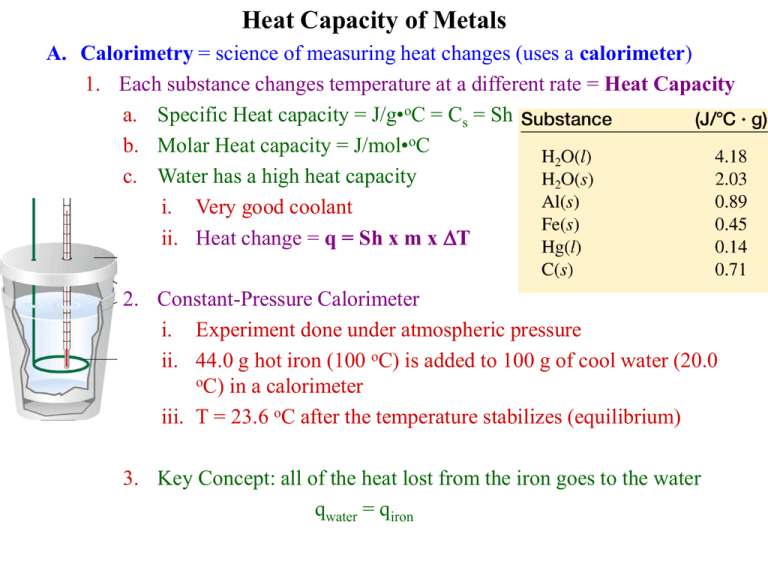
Heat Capacity of Metals A. Calorimetry = science of measuring heat changes (uses a calorimeter) 1. Each substance changes temperature at a different rate = Heat Capacity a. Specific Heat capacity = J/g•oC = Cs = Sh b. Molar Heat capacity = J/mol•oC c. Water has a high heat capacity i. Very good coolant ii. Heat change = q = Sh x m x DT 2. Constant-Pressure Calorimeter i. Experiment done under atmospheric pressure ii. 44.0 g hot iron (100 oC) is added to 100 g of cool water (20.0 oC) in a calorimeter iii. T = 23.6 oC after the temperature stabilizes (equilibrium) 3. Key Concept: all of the heat lost from the iron goes to the water qwater = qiron qwater qiron Shwater mwater DTwater Shiron miron DTiron (4.18 J / gK )(100 g )(3.6 K ) ( x)( 44.0 g )(76.4 K ) x (4.18 J / gK )(100 g )(3.6 K ) 0.448 J / gK (44.0 g )(76.4 K ) B. Procedure 1. Use water bath on a hot plate to heat metal in a test tube, record Temp 2. Record the initial temperature and weight of your calorimeter (calorimeter = two styrofoam cups) 3. Add the hot metal to the calorimeter and record the final (highest) Temp 4. Weigh the calorimeter + metal 5. Repeat two more times to get an average specific heat of the metal 6. Repeat the whole thing on a second metal 7. Recycle the cups and the metal: don’t throw them away C. Deviation from the mean = |Shi – Shavg| Incident: Broken Mercury Thermometer











