Questions for Specific Heat Capacity Lab Questions:
advertisement

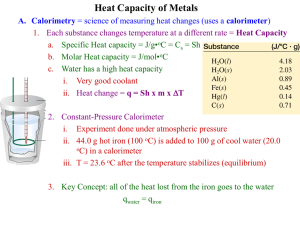
Questions for Specific Heat Capacity Lab Questions: 1. Explain the difference of energy change when Q and ΔT are positive and when they are negative. 2. What had the heat that was lost? 3. What absorbed the heat that was lost? 4. Why can’t the energy transfer be found of the calorimeter and water at the same time? 5. In order for the specific heat of the sample to be found, list what is needed to be known? 6. What overall purpose of this lab? Can you sum it up it one sentence? 7. Explain five errors that occurred in the lab. 8. What is the purpose of the outer shell of the calorimeter cup and insulating ring? 9. Explain why the energy transferred as heat into the calorimeter and the water is (should be) equal to the energy transferred as heat from the metal. 10. How is the temperature change of the calorimeter and the water within the calorimeter affected by the specific heat capacity of the metal? Did a metal with a high specific heat capacity raise the temperature of the water and the calorimeter more or less than a metal with a low specific heat capacity?