Classifying Changes of Matter: Physical vs
advertisement

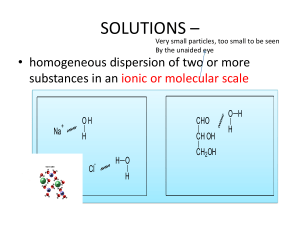
Classifying Changes of Matter: Physical vs. Chemical Physical Change Physical A change in matter from one form to another without changing its chemical properties (most can be reversed) No change in atoms/molecules Physical Change • Examples = Change in state or phase change Dissolving Compressing Light emission/absorption Electrons passing through metals Physical Change More Examples = • boiling of a liquid • melting of a solid • dissolving a solid in a liquid to give a homogeneous mixture • making a solution 2 1 Chemical Change Chemical Bonds are made / broken Change in oxidation states Cl-1 Cl-1 Li +1 Examples of Chemical Change •Burning hydrogen (H2) in oxygen (O2) gives H2O. • Chemical change or chemical reaction — transformation of one or more atoms or molecules into one or more different molecules. Sure Signs of a Chemical Change • Heat • Light • Gas Produced (not from boiling!) • Precipitate – a solid formed by mixing two liquids together Physical vs. Chemical Change • Examples: ▫ melting physical ▫ flame produced chemical ▫ paper cut ▫ dissolving salt in water ▫ tarnishes in air physical physical chemical Physical vs. Chemical Change • Examples: ▫ rusting iron chemical ▫ mixing mud and water physical ▫ burning a log ▫ freezing water ▫ grinding spices chemical physical physical • A difference between physical change and chemical change is that 1. chemical change involves energy while physical change does not. 2. physical change involves energy while chemical change does not. 3. different kinds of molecules are present after a physical change but not after a chemical change. 4. different kinds of molecules are present after a chemical change but not after a physical change In making a pizza, which process involves a chemical change? 1. Mixing spices for the sauce 2. Slicing pepperoni for the topping 3. Spreading cheese on the pizza 4. Baking the dough to form the crust