1-1 Review & Reinforcement
advertisement

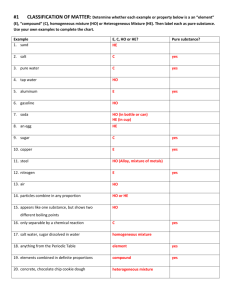
1-1 Review & Reinforcement Name_________________________ Matter/Physical & Chemical Changes Period_______Date______________ Complete the following sentences by filling in the appropriate word. 1. ________________________is anything that has mass and volume. 2. The two states of matter that occupy a definite volume are _______________& _______________. 3. __________________ & ___________________ are the other two states of matter. 4. ___________________ changes alter the identity of a substance, whereas _________________ changes do not. Identify whether each of the following changes is a physical change or a chemical change. Write a “P” on the line for a physical change and a “C” for a chemical change. 5. _____ water boiling 12. _____ iron rusting 19. _____ butter melting 6. _____ alcohol evaporating 13. _____ wood rotting 20. _____ glass breaking 7. _____ leaves changing color 14. _____ mowing the lawn 21. _____ baking a cake 8. _____ magnetizing a nail 15. _____ digestion of food 22. _____ cutting paper 9. _____ lighting a match 16. _____ stretching a rubber band 23. _____ burning paper 10. _____ dissolving sugar in water 17. _____ melting glass 24. _____ paint fading 11. _____ a puddle dries up 25. _____ cracking an egg 18. _____ a pond freezes over Determine which of the following are True “T” or false “F”, and for each false statement, substitute for the underlined word(s), the word(s) which makes the statement correct. 26. _____________________ A change in state indicates that a chemical change has occurred. 27. _____________________ A change in composition indicates that a chemical change has occurred. 28. _____________________ A change in shape indicates that a physical change has occurred. 29. _____________________ Both physical & chemical changes involve energy changes. 30. _____________________ The burning of wood is a physical change. 31. _____________________ When ice cubes melt, a chemical change takes place. 2-3 Review-matter 32. _____________________ When mercury (II) oxide breaks down into its elements, mercury and oxygen, it has undergone a physical change. 33. _____________________ The separation of a mixture into its parts is a physical change. 34. _____________________ The melting of iron is a chemical change. 35. _____________________ A glowing candle is an example of a physical change. 36. _____________________ Droplets of water forming on the outside of a glass of soda is a chemical change. 37. _____________________ Formation of water from its elements is a physical change. Answer each of the following questions in the space provided. 35. What is the relationship between the kinetic energy of molecules and their physical state? _______________________________________________________________________________ _______________________________________________________________________________ 36. How could you determine whether the dissolving of sugar in water is a physical or chemical change? _______________________________________________________________________________ _______________________________________________________________________________ 37. Can a chemical change be accompanied by a physical change? Why? _______________________ ______________________________________________________________________________ 38. A salt water mixture can be separated by distillation. Is that a physical or chemical change? Why? ______________________________________________________________________________ ______________________________________________________________________________ 39. A bottle of milk turns sour. Is this a physical or chemical change? Why? ___________________ ______________________________________________________________________________ 40. Helium is an inert gas that does not react with other substances to form compounds. Would it be correct to say that Helium has no chemical properties? Explain your answer. ______________________________________________________________________________ ______________________________________________________________________________ 2-3 Review-matter