The Integrated Rate Law
The Integrated Rate Law
Section 13.4
The Integrated Rate Law
Integrated Rate LawA relationship between the the concentrations the reactants and time
To keep it simple, a single reactant decomposes into products
A-->products
Scientists use this law to estimate the time it will take to rid the atmosphere of Chloroflourocarbons (CFC’s)
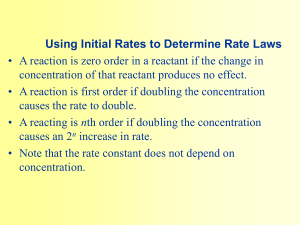
First Order Reaction
In a first order simple reaction, the rate is proportional to the concentration of A
Rate= k [A] or Δ[A]/Δ t = k [A] since rate=Δ[A]/Δ t
This is known as the differential rate law as well
Using calculus, this equation can be integrated to ln[A] t
/ [A]
0
=kt
First order reaction
ln[A] t
=kt +ln[A]
0
This equation is the same as a straight line
A first order simple reaction will always have a negative correspondence between time and ln[A]
Example
Find the constant if the molarity of SO
2
Cl
2 is 0.084 at 600 seconds and
0.100 M initially?
ln([A] t
/[A]
0
)=kt ln(.084/.100)=k (600) k =2.91x10
-4 s -1
Second Order Reactions
When the reaction is second order, the rate is proportional to the square of the the concentration of A
Rate= k [A] 2 or = k [A] 2
Again, with calculus, this equation can be integrated to become:
1/[A] t
= kt +1/[A]
0
Second Order Reactions
The graph of a second order reaction is also a line
1/[A] t
= kt +1/[A]
0
You have to plot the inverse of the concentration of the reactant as a function of time
Example
If the rate constant of a reaction is 0.225 M -1 •s -1 and the initial molarity is
0.010M, what molarity would be completely decomposed at 300 seconds?
1/[A] t
= kt +1/[A]
0
1/[A] t
=(0.225)(300)+1/0.010
[A] t
=5.97x10
-3
Zero Order Reaction
In a zero order reaction, the rate is proportional to a constant
Rate= k [A] 0 = k or = k
Once more, this can be integrated into the zero-order integrated law
[A] t
=kt +[A]
0
The graph of a zero order function is a line as well
Half-Life
Half Life -Time it takes required for the concentration of a reactant to fall to its initial value
There are three types of half lives
First Order Reaction Half Life
Second Order Reaction Half Life
Zero Order Reaction Half Life
LO 4.3 The student is able to connect the half-life of a reaction to the rate constant of a first-order reaction and justify the use of this relation in terms of the reaction being a first-order reaction. [See SP 2.1, 2.2]
First Order Reaction Half Life
To get the First-Order Half Life equation, start with the original first-order equation
Then substitute t
1/2 for t and ½[A]
0 for [A]
0
Ln(½[A]
0
)/[A]
0
=ln1/2=kt
1/2
Solve for t
1/2
(use -0.693for ln1/2) t
1/2
=0.693/ k
Second Order Reaction Half Life
Once again, start with the original equation for a second order reaction
Substitute t
1/2 for t and 1/2[A]
0 for [A] t
1/(½[A]
0
)= kt
1/2
+1/[A]
0
Solve for t
1/2 t
1/2
=[A]
0
/2 k
Zero Order Reaction Half Life
Start with the integrated zero order reaction equation
Substitute the variables
½[A]
0
=kt
1/2
+[A]
0
Solve for t
1/2 t
1/2
=[A]
0
/2 k
Example
Find the half life of a zero-order half life equation when the original molarity is
0.250M and the constant is 0.042M ⋅ sec -1 .
t
1/2
=[A]
0
/2 k t
1/2
=0.250/2(0.042) t
1/2
=2.98sec