Organic Reactions
advertisement

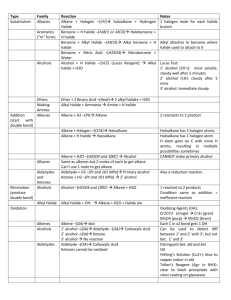
So far in this unit we have discussed hydrocarbons and their isomers We have also learned about organic compounds with different functional groups Throughout this we have learned naming rules for each Now we are going to focus on several types of organic reactions There are many types of organic reactions We are only going to focus on a few. › Substitution › Elimination › Addition › Esterification Any reaction in which one atom is replaced by another Used to place a halogen onto an alkane The products are always a halocarbon and the acid of the halogen Reaction requires ultraviolet light to initiate the reaction › This provides the energy needed to form the excited state ethane + chlorine chloroethane + hydrochloric acid The Cl atom will replace a H atom resulting in an alkyl halide and the acid of the halogen (hydrochloric acid) You can also have an alcohol react with a halogen resulting in an alkyl halide and water Ethanol + hydrochloric acid chloroethane + water Any reaction in which atoms are eliminated from another molecule Done in three ways: › Elimination of H2 › Elimination of HX › Elimination of H2O Called dehydrogenation Occurs in the presence of a base (eg. NaOH) and heat ethane ethene + hydrogen Alkyl halides undergo elimination reaction This is known as dehydrohalogenation Occurs in the presence of a base Base extracts H+ and X- will leave; resulting in double bond formation Alcohol can undergo elimination via the loss of water Known as dehydration Acid protonates the OH group, water leaves and C+ remains behind b) An adjacent H+ leaves next leaving electron pair to form double bond a) Takes place with unsaturated compounds which are usually more reactive than saturated compounds Takes place with double and triple bonds Two atoms are added across the electron rich bond Four types of addition: › › › › Addition of X2 (halogen) Addition of H2 Addition of H2O Addition of HX (hydrogen halide) Normally occurs in dissolved solvents such as CCl4 Alkenes form dihaloalkanes Alkynes produce dihaloalkenes or tetrahaloalkanes ethene + chlorine 1,2-dichloroethane Catalysts used such as Pt, Pd or Ni Known as hydrogenation Alkene becomes alkane Alkyne becomes alkene or alkane ethene + hydrogen ethane ethyne + hydrogen ethyne + (2 mol) hydrogen ethene ethane Occurs in the presence of an acid Know as hydration Alkene becomes alcohol Alkyne produces ketone or aldehyde ethene + water ethanol HX = HCl or HBr or HI (NOT HF) Alkene becomes alkyl halide Alkynes form monohalo alkenes or dihalo alkanes (with halogen on the same C) Rule for adding H and X › The halogen (X) will always add to the more substituted carbon atom – the carbon that is bonded to the most carbon atoms 1-propene + hydrobromic acid 2-bromopropane Step1: 1-propyne + hydrobromic acid 2-bromo-1-propene Step 2: 2-bromo-1-propene+ hydrobromic acid 2,2-dibromopropane Alcohol + organic acid water + ester Used to make perfumes, scents and flavours Combination reaction which involves dehydration Alcohol becomes the alkyl group and the acid becomes -oate propanol + ethanoic acid propyl ethanoate + water