Alkenes and Alkynes Chapter 1.2
advertisement

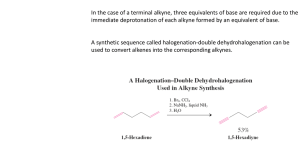
Alkenes and Alkynes Chapter 1.2 Unsaturated Hydrocarbons Alkenes and alkynes are unsaturated hydrocarbons • An alkene is a hydrocarbon that contains at least one carbon-carbon double bond • An alkyne is a hydrocarbon that contains at least one carbon-carbon triple bond ethyne ethene Alkanes, alkenes and alkynes are all aliphatic hydrocarbons which means their strucutres are based on straight or branched chains or rings of carbon atoms Naming Alkenes and Alkynes • Naming alkenes and alkynes is very similar to naming alkanes • The parent chain must be numbered so that the double or triple bonds are on the lowest numbered carbons possible • The suffixes –ene and –yne are used • If there is more than one multiple bond indicate this using -diene or –triene • A number may also be necessary to indicate which carbon on the parent chain or ring the multiple bond occurs on • For example, the molecule below would be named 3,3-dimethylbut-1-ene Practice Name the following a) b) c) d) Redundancy revisited What is wrong with the name prop-1-ene? Stereoisomers Revisited • Like cycloalkane ring structures, alkenes and alkynes have restricted rotation because of the multiple bonds • Alkenes have two groups attached to each double bonded carbon and their position is fixed due to the lack of rotation in the double bond, thus cis-trans isomerism is possible • Imagine that the molecule is divided into two sides along the double bond • The cis isomer will have two matching groups on the same side of the double bond • The trans isomer will have the matching groups on opposite sides of the double bond Practice • Example: • Name the following a) b) c) d) Properties of Alkenes and Alkynes • A functional group is a group of atoms within a molecule that determines the properties of the molecule • Multiple bonds are considered to be functional groups because they affect the properties of the molecules that contain them • For example, multiple bonds are less stable than single bonds between carbon atoms, thus alkenes and alkynes are more reactive than alkanes Reactions of Alkenes and Alkynes • Like alkanes, alkenes and alkynes undergo combustion reactions • Alkenes and alkynes also undergo addition reactions • An addition reaction is a reaction in which the atoms from one molecule are added to another molecule to form a single molecule • The four addition reactions that alkenes and alkynes undergo are: 1) Hydrogenation 2) Halogenation 3) Hydrohalogenation 4) Hydration Reactions of Alkenes and Alkynes 1) Addition – Hydrogenation • Hydrogen is added to the alkene or alkyne and reacts with the double bond to form a more saturated product • This reaction takes place in the presence of a catalyst Reactions of Alkenes and Alkynes 1) Addition – Hydrogenation + H2 catalyst catalyst + excess H2 Reactions of Alkenes and Alkynes 2) Addition – Halogenation • A halogen (such as bromine or chlorine) is added to the alkene or alkyne • The halogenation of an alkene produces an alkyl halide + Br2 Reactions of Alkenes and Alkynes 2) Addition – Halogenation • The halogenation of an alkyne produces a halogenated alkene or, if excess halogen is present, an alkyl halide Reactions of Alkenes and Alkynes 3) Addition – Hydroalogenation A hydrogen halide (such as hydrogen chloride or hydrogen bromide) is added to an alkene or an alkyne But wait! There’s a PROBLEM! Predict the products of the hydrohalogenation of but-1-ene + HBr Markovnikov’s Rule When a hydrogen halide or water is added to an alkene, the hydrogen atom generally bonds to the carbon atom within the double bond that already has more hydrogen atoms bonded to it. Reactions of Alkenes and Alkynes 4) Addition – Hydration • Water is added to an alkene or an alkyne • This reaction produces a type of organic compound called an alcohol • Alcohols contains a hydroxyl group (-OH) • This reaction takes place in the presence of a catalyst • Markovnikov’s rule may be used in hydration reactions as well catalyst Reactions of Alkenes and Alkynes A. Combustion B. Addition 1) Hydrogenation 2) Halogenation 3) Hydrohalogenation 4) Hydration HOMEWORK Required Reading: p. 18-27 Questions: p. 21 #1-2 p. 23 #1-3 p. 26 #1-2 p. 27 #1-10 Remember to ask for extra help if you need it!!