Four Types of Organic Molecules
advertisement

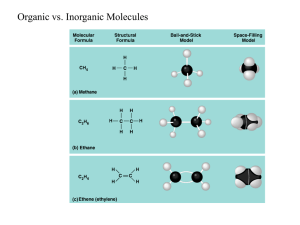
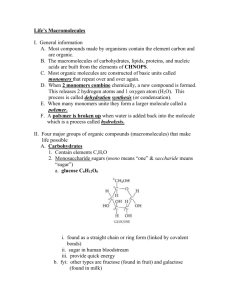
Four Types of Organic Molecules Made by cells Contains carbon Importance of Carbon Although cells are 70-95% water, the rest is composed mainly of carbon compounds. Proteins, carbohydrates, DNA, and other molecules are compounds of carbon bonded to other elements. Carbon often bonds to H, O, N, S, and P in organic compounds. Properties of Carbon Has four valence electrons; can form covalent bonds with four other atoms (tetravalence) Carbon bonded to four atoms forms a tetrahedron-shaped molecule. Carbon can form single, double, or triple bonds with other atoms. Carbon chains form the backbone of most organic molecules. Chains can be straight, branched, or arranged in closed rings. Hydrocarbons contain carbon and hydrogen only, and are hydrophobic. H—C and C—C bonds are nonpolar. Hydrocarbons make up fossil fuels, and parts of cellular organic molecules such as fats and phospholipids. 1. Carbohydrates- used as fuel and building material 2. Lipids-energy storage 3. Proteins-structure, movement, enzymes 4. Nucleic acids-store and transmit hereditary information. All four are macromolecules because of their large size. The largest Macromolecules are called polymers Created by linking smaller subunits called monomers. Dehydration Monomers are linked together to form polymers through dehydration reactions, which remove water Monomers are linked together by covalent bonds Short polymer Dehydration reaction Longer polymer Unlinked monomer Hydrolysis Polymers are broken apart by hydrolysis, the addition of water Breaks covalent bonds between monomers Hydrolysis 1. Carbohydrates monosaccharides - one ring sugars glucose, galactose and fructose * A 6-carbon sugar * Found in peas. * A 6-carbon sugar * The sugar in our blood * A 5-carbon sugar * the sugar that sweetens fruit Diabetes Disease characterized by high levels of blood glucose resulting from defects in insulin production Monitored with blood glucose device. disaccharides - two monosaccharides (2 ring sugar) Glucose + fructose = Sucrose (table sugar) Glucose + galactose = Lactose (milk sugar) Glucose + glucose = Maltose (malt sugar) Lactose intolerant If the enzyme lactase is not present, the body is unable to break down lactose. Allowing it to reach the large intestines. Normally, sugars do not reach the large intestine. This is what causes a stomach ache! polysaccharides - long chains of repeating units of monosaccharides, these are energy storing molecules or structural molecules Energy: starch (plants produce for a storage molecule.) glycogen (storage molecule in muscle and liver cells.) Structural: chitin (used by insects and crustaceans to build an exoskeleton.) cellulose (plants produce for cell wall construction.) indigestible because we lack enzymes to break it down. Starch, Cellulose & Glycogen All the sugars are oriented in the same direction Branched or "forked" Every other sugar molecule is "upside-down Review Q: Polymers of carbohydrates are all synthesized from monomers by A) the joining of disaccharides. B) hydrolysis. C) dehydration synthesis. D) ionic bonding between monomers E) cohesion. 2. Protein Made of a long chain of amino acids Make up 50% of cellular dry weight Amino acids (building blocks) Have an amino group and a carboxyl group Also a chemical group symbolized by R Amino group Carboxyl group – Dehydration reaction links the carboxyl group of one amino acid to the amino group of the next amino acid – The covalent linkage resulting is called a peptide bond Carboxyl group Amino group Dehydration reaction Peptide bond Dipeptide Amino acid Amino acid Proteins function as… Support keratin for hair and nails & collagen for ligaments, tendons, skin Proteins function as… Enzymes to speed up reactions Example: Amylase is an enzyme in saliva that breaks starch into glucose monomers. Saliva Saliva Identification - Amylase Proteins function as… Transport across cell membranes Hemoglobin Defense from infection Antibodies Hormones Insulin Insulin is a hormone that helps your body use glucose. Protein structure is key to their ability to function. **A protein can be denatured, heat causes it to lose its shape, and its functionality.. Review Q: The linkage between the monomers of proteins are identified as A) Peptide bonds B) Glycosidic linkages C) Ionic bonds D) Covalent bonds E) Ester linkages Review Q: Which two functional groups are always found in amino acids? A) Carboxyl and sulfhydryl B) Carbonyl and carboxyl C) Carboxyl and amino D) Alcohol and aldehyde E) Ketone and sulfhydryl 3. Nucleic Acids Stores information Two types: DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Nucleotides are the building blocks of Nucleic acids. 4. Lipids Hydrophobic – Will not mix with water Types of Lipids: Phospholipids - form cell membranes The polar heads are towards the water, the nonpolar tails are on the inside of the cell. Steroids cell messengers examples: testosterone, estrogen Waxes protection & waterproofing Triglycerides fats and oils fats are made of chains of fatty acids Saturated & Unsaturated Saturated - no double bonds solid at room temperature Unsaturated - have double bonds liquid at room temperature Review Q: Which macromolecule is the main component of cell membranes? A) Glucose B) Steroids C) Carbohydrates D) Phospholipids E) DNA Review Q: Which of the following macromolecules below could be structural parts of the cell, enzymes, or involved in cell movement or communication? A) Nucleic acids B) Proteins C) Lipids D) Carbohydrates E) Minerals