File - Kristen E. Berglund
advertisement

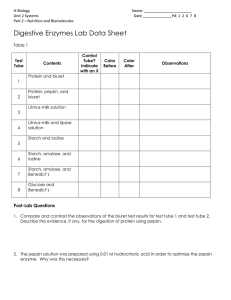
Kristen Berglund General Biology Laboratory 191 S. Connolly, J. Dean, W. Gilliland, C. Martineau, and K. Sheehan October 15, 2012 Lab 5: Enzyme Activity Introduction: In a chemical reaction, enzymes work as a catalyst and speed up the reaction. Each enzyme, however, works on a specific substrate, or group of molecules. Since an enzyme is a protein, the structure of the enzyme determines the function. The enzyme amylase helps to break down starch into smaller sugars that helps to power cellular work. In this experiment, the goal was to find out whether or not a fungal Amylase could replace a natural, human amylase. Then, the experiment was taken even further and the elements of pH and temperature were involved as factors to measure the effect on the rate of starch digestion by fungal amylase. The general question was as to whether or not the activity of amylase would be affected by pH and temperature in separate experiments. It was predicted that at body temperature, starch digestion by fungal amylase would decrease and that the further the pH is from its optimum pH, the lower the enzyme’s activity. The hypotheses were that amylase would have an optimal pH where it exhibits greatest activity and that temperature has an effect on the rate of starch digestion in fungal amylase. In the last experiment there was a biological hypothesis, a null hypothesis, and an alternative hypothesis. The biological hypothesis is a proposed explanation where there is significant change while the null hypothesis is essentially the opposite. In the null hypothesis, there is no significant change. An alternative hypothesis is where there is significant change and it relates two variables. For this experiment, the alternative hypothesis was that there is a significant difference in amylase activity at room temperature versus at body temperature. In order to test these hypotheses, amylase activity was measured by the change in the amount of the substrate (starch) or the product (maltose) by using a spectrometer and an iodine test. The spectrometer quantifies the results of the iodine results to measure the amount of starch left after an amylase reaction. Methods: First, a standard curve is measured by using the spectrometer to measure the absorbance of starch solutions of a known concentration treated with the IKI iodine solution. There are three test tubes already prepared to construct the standard curve. First, set the desired wavelength on the spectrometer. Zero the spectrometer by turning the dial until it reads 0.00. Press the mode button until the absorbance option is lit. Now blank the spectrometer by wiping off the prepared yellow test tube and turn the dial until the absorbance reads 0.000. This means that the spectrometer is blanked and the other samples are ready to be tested for absorbance values of starch solutions. Wipe down the unknown sample, put that in the chamber and read the absorbance at the set wavelength (580 nm). Construct a standard curve by using the rest of the class data. This was to test how the initial starch concentration minus the final starch concentration was equal to the amount of starch digested. The second part of the experiment was to test the factors of pH and temperature on starch digestion by fungal amylase then the construct a statistical analysis using the standard deviation, standard error, and critical values. In the sample experiment, the pH was used to determine whether or not Amylase had an optimum pH where it exhibited the greatest activity. In this experiment, six test tubes ranged in pH levels. The first test tube should be a negative control, where it is at room temperature, no starch, 1 mL amylase and 2 mL buffer with a pH of 7.5. The sixth test tube should be the positive control where it is at room temperature, 1 mL starch, no amylase, and have 2 mL of the buffer with a pH of 7.5. The test tubes in between should only vary in pH levels. Each of the four remaining test tubes are at room temperature, have 1 mL of starch, and 1 mL of amylase. However, test tube 2 should have 1 mL of the buffer with a pH of 5.5. Test tube 3 should have a 1mL buffer at 6.5. Test tube 4 should have 1 mL buffer at 7.5. Finally, test tube 5 should have 1 mL buffer with a pH of 8.5. After letting the reaction run for ten minutes, 1 mL of iodine should be added to each test tube in order to stop the amylase. Then add 3 mL of water to each test tube and immediately put in the spectrometer to be read. The data for each test tube should be collected in order to indicate the optimum level of pH in the Amylase activity. Finally, the last part of the experiment was to test whether or not temperature has an effect on starch digestion by fungal amylase. For the experimental design, the negative control will be at 22˚C with 0 mL starch, 1 mL amylase, and 2 mL buffer. The first experimental tube (test tube 2) will be at 22˚C, have 1 mL starch, 1 mL amylase, and 1 mL buffer. The 2nd experimental test tube (test tube 3) will be at 37˚C with 1 mL starch, 1 mL amylase, and 1 mL buffer. Finally the positive control should be at 22˚C and have 1 mL starch, 0 mL amylase, and 2 mL buffer. Label four test tubes with two being the negative and positive control. Add 1 mL 0.4 mg/mL starch solution to all but the negative control. Add an adequate amount of buffer from the flask that will be intubated at the correct temperature. For example, use the buffer in the 37˚C bath for reactions that will be run at 37˚C. Add 1 mL of Amylase to each tube and immediately move any reactions at a specific temperature to the correct temperature water bath. Allow the reaction to sit in the bath for ten minutes exactly. Then, when the ten minutes are up, add 1 mL IKI to each test tube to stop the reaction. Add three drops of water to the negative control only. Invert the negative control tube and place in the spectrometer and zero the balance. This will act as the blank tube. Add three mL water to the second test tube, invert, and place in the spectrometer and read the absorbance. Repeat with the remaining test tubes to read the absorbance. Absorbance should be read at 580 nm and record Data. Use the standard curve from earlier to estimate the concentration of the starch remaining based on the absorbance. Subtract this value from the initial starch concentration for the values of the starch digested and record results. Collect class data and calculate the average, standard deviation and standard error for each condition. Then using this data and the T-test critical values, determine if the null hypothesis is supported or failed to be rejected. The ttest is a statistical analysis tool that compares the mean values between two sets of data and is a ratio. There are 14 degrees of freedom in this procedure. Results: Table 6: Class Data: Starch Digested (mg/mL) Tube Tube 2 Tube 3 Temperature 22˚C 37˚C Group 1 0.27 0.38 Group 2 0.32 0.37 Group 3 0.35 0.39 Group 4 0.3 0.375 Group 5 0.275 0.395 Group 6 0.35 0.34 Group 7 0.325 0.250 Group 8 0.5 0.35 Average 0.28 0.356 Standard Deviation Standard Error 0.0977 0.468 0.0345 0.0165 This table shows the class data collected form the starch digested when temperature would affect the fungal Amylase activity. Figure 1: Graph of Group Data: Starch digested (mg/mL) 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 22˚C 37˚C This bar graph shows the collective class data for the two test tubes at their specific temperatures. Results: After the temperature portion of the experiment, a statistical test called a t-test was used in order to assess whether or not the results were valid answers. There were 14 degrees of freedom in this experiment. The results show that at 22˚C, the starch digested was lower than at 37˚C. Using the standard deviation and a t-test, the t-statistic of 1.990 was outside the p-value, which was from 0.010.05. Discussion: After the temperature portion of the experiment, the null hypothesis failed to be rejected. The t-test proved that the data was not significant as it was outside the p-value. This means that there is not significant data to support the claim that fungal amylase could be a healthy alternative to human amylase. In this experiment, replication was necessary in order to prove that the enzyme used was not faulty and could be used many times with the same results. The procedure should not be altered as it works very smoothly for many in the class to reach the same results. Only two factors were tested with enzyme activity, temperature and pH level. However, perhaps another experiment could be performed where the concentration of the substrate and of the enzyme concentration are factors. Many of the results throughout the class had consistent data, however, faulty data could easily be resulted from a dirty test tube, a faulty spectrometer, or even finger marks on a test tube. After doing this experiment, it should be reported back to the biotechnology company that fungal amylase has not yet been supports as a healthy alternative to natural, human amylase and more tests are needed.