Ligand E o /V
advertisement

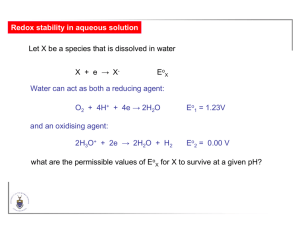
Chapter 5 – Oxidation and Reduction Consider: +6 2- 0 SO4 + 2CH2O + 2H+ -2 +4 → H2S + 2CO2 + 2H2O reduction oxidation A redox reaction is the outcome of a reduction and an oxidation half reaction SO42- + 8e + 10H+ → H2S + 4H2O CH2O + H2O → CO2 + 4e + 4H+ By convention, redox reactions are written as REDUCTION reactions SO42- + 8e + 10H+ → H2S + 4H2O (2) CO2 + 4e + 4H+ → CH2O + H2O (3) SO42- + 2CH2O + 2H+ → H2S + 2CO2 + 2H2O (1) The overall equation is the difference of the chemical equations for the two half reactions; but number of electrons gained and lost must be the same (1) = (2) + 2 x (-3) Gibbs energy for reaction = difference of the standard Gibbs energies for the half reactions Because half reactions must always occur in pairs, only differences in G have any physical meaning Chose one half reaction as a standard, and measure all Gibbs energies relative to this half reaction 2H+(aq) + 2e → H2(g) Go = 0 Measure G for each half reaction by coupling to a half reaction of known G (such as the H+|H2 couple) in a galvanic cell Fig. 5.1 For the reaction cAA + cBB → cCC + cDD let the reaction advance by an infinitesimally small amount d. Greek - Xi Then dnA dnB dnC dnD = = = = -cAd -cBd +cCd +cDd The chemical potential of a pure substance is defined as G n T , p For the reaction cAA + cBB → cCC + cDD dG = CdnC + DdnD + AdnA + BdnB = CcCd + DcDd - AcAd - BcBd = (CcC + DcD - AcA - BcB)d In general dG cJ J d J dG cJ J d J G cJ J G J T , p Hence dG = G d and the maximum work that the reaction can do as it proceeds through an extent d at constant T and p is dw = G d As the reaction advances by d then nd electrons travel from the anode to the cathode. e cathode anode The total charge transferred is therefore -neNAd = -nFd where F = eNA is the Faraday constant Since Work done Potential Difference, E = charge transferred dw E= -nFd dw nEFd and since we had that dw = G d G = -nFE and under standard conditions Go = -nFEo Since by definition 2H+(aq) + 2e → H2(g) Go = 0 it follows immediately that the standard reduction potential is 2H+(aq) + 2e → H2(g) Eo = 0.00 V Given SO42- + 8e + 10H+ → H2S + 4H2O Eo = -0.221 V CO2 + 4e + 4H+ → CH2O + H2O Eo = -0.072 V SO42- + 2CH2O + 2H+ → H2S + 2CO2 + 2H2O SO42- + 8e + 10H+ → H2S + 4H2O Eo = -0.221 V 2CH2O + 2H2O → 2CO2 + 8e + 8H+ Eo = 0.072 V Remember that you don’t double Eo! Because G = -nFEo, doubling n will have doubled G already SO42- + 2CH2O + 2H+ → H2S + 2CO2 + 2H2O Eo = -0.221 V + 0.072 V = -0.149 V Go = -nFEo = -8 x 96 485 C mol-1 x 0.072 J C-1 = 115 kJ mol-1 G > 0 reaction is not spontaneous If Eo < 0, the reaction is not spontaneous For a spontaneous reaction, Eo > 0 The electrochemical series lists half reactions in order of Eo. The more positive Eo the stronger the species is as an oxidising agent See Table 5.2 Example Calculate Eocell and G for the oxidation of copper by nitric acid Recall that for the general reaction aA + bB → cC + dD aCc aDd Q a b aA aB where aIi i [I]i and i is the activity coefficient (=1 for dilute solutions) Q is called the reaction quotient and Q = K, the equilibrium constant, only when a system is at equilibrium G = Go + RT ln Q G = Go + RT ln Q -nFE = -nFEo + RT ln Q RT E = E ln Q nF o E = Eo 2.303 RT log Q nF Nernst Equation or 0.0592 V E = E log Q (T= 298 K) n o At equilibrium, Q = K, and E = 0 because G = 0 So E = Eo RT ln Q nF RT 0 = E ln K nF nFEo ln K = RT o Examples How does the reaction O2 + 4H+ + 4e → 2H2O Eo = 1.23 V depend on pH? Hence determine Eo for the reaction O2 + 2H2O + 4e → 4OH– Example 2 Given: Br2 + 2e → 2Br– Cl2 + 2e → 2Cl– Eo = 1.065 V Eo = 1.359 V calculate the equilibrium constant at standard thermodynamic conditions for the reaction Br2 + 2Cl– → Cl2 + 2Br– OVERPOTENTIALS Since Co2+ + 2e → Co 2H+ + 2e → H2 Eo = -0.277 V Eo = 0.000 V cobalt metal in contact with acid should release hydrogen vigorously Co + 2H+ → Co2+ + H2 Eocell = 0.277 V The reaction does occur but is slow. Thermodynamics tells us nothing about the speed (kinetics) of a reaction. Empirical observation: reduction of one couple by another occurs at a significant rate only when the difference in potentials of the two couples exceeds a characteristic value known as the overpotential. To reduce H+ to H2: need an overpotential of about -0.6 V To oxidise H2O to O2: need an overpotential of about +0.6 V Example Assuming that we can maintain an exposed surface, will Al undergo rapid oxidation in water at pH 7? Al3+ + 3e → Al 2H+ + 2e → H2 Eo = -1.66 V Eo = 0.00 V Example 2 What minimum potential must a couple have to be able to oxidise water at pH 7? Xn+ + ne → X O2 + 4H+ + 4e → 2H2O Eo = x V Eo = 1.23 V Redox stability in aqueous solution Let X be a species that is dissolved in water X + e → X- EoX Now since: O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V and: what are the permissible values of EoX for X to survive at a given pH? O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V 0.0592 V E = E log Q n o 1 E = 1.23 V 0.0592 V x pH as we have seen before Similarly for 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E = Eo 2 0.0592 V log Q n E = 0.00 V 0.0592 V x pH Let’s look specifically at pH 7 O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V E = 1.23 V 0.0592 V x pH EpH7 = 1.23 - 0.0592 x 7 = 0.82 V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V E = 0.00 V 0.0592 V x pH EpH7 = 0.00 - 0.0592 x 7 = -0.41 V O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V EpH7 = 0.82 V EpH7 = -0.41 V X + e → X- EoX Suppose EoX < -0.41 V. For example, let EoX = -0.50 V 2X- → 2X + 2e 2H3O+ + 2e → H2 + 2H2O 2H3O+ + 2X- → 2X + 2H2O + H2 0.50 V -0.41 V 0.09 V The reaction is spontaneous and X- will not survive. It will be oxidised by water. O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V EpH7 = 0.82 V EpH7 = -0.41 V X + e → X- EoX Suppose EoX > 0.82 V. For example, let EoX = 0.90 V 4X + 4e → 4X2H2O → O2 + 4H+ + 4e 2H2O + 4X → 4X- + O2 + 4H+ 0.90 V -0.82 V 0.08 V The reaction is spontaneous and X will not survive. It will by reduced by water. Finally suppose -0.41 V < EoX < 0.82 V. For example, let EoX = 0.10 V 4X + 4e → 4X2H2O → O2 + 4H+ + 4e 2H2O + 4X → 4X- + O2 + 4H+ 0.10 V -0.82 V -0.72 V The reaction is not spontaneous. 2X- → 2X + 2e 2H3O+ + 2e → H2 + 2H2O 2H3O+ + 2X- → 2X + 2H2O + H2 The reaction is not spontaneous. -0.10 V -0.41 V -0.51 V O2 + 4H+ + 4e → 2H2O Eo1 = 1.23V 2H3O+ + 2e → 2H2O + H2 Eo2 = 0.00 V EpH7 = 0.82 V EpH7 = -0.41 V 0.82 V For a species to be stable in water, its redox potential must lie between these values at pH 7. This is sometimes referred to as the Electrochemical Window -0.41 V Recall, however, that the overpotential may make species with Eo values outside the electrochemical window kinetically stable – i.e., they can exist in solution because the kinetics of their reaction with water are slow By applying the Nernst equation, we can get the stability field of water at any pH. E = 1.23 V 0.0592 V x pH E = 0.00 V 0.0592 V x pH Figure 5.3 Example Is Co(III) (a) thermodynamically and (b) kinetically stable in water at pH 0 and at pH 7? O2|H2O Co(III) + e → Co(II) Eo = 1.92V 1.23 V 0.82 V pH 0 pH 7 0.00 V -0.41 V H+|H2 Aluminium should react spontaneously with water at pH 7: Al3+ + 3e → Al O2|H2O Eo = -1.66 V H+|H2 2Al → 2Al3+ + 6e 6H+ + 6e → 3H2 Eo = 1.66 V EpH7 = -0.41 2Al + 6H+ → 2Al3+ + 3H2 Eo = 1.25 V pH 7 0.82 V -0.41 V It doesn’t because the surface is passivated by the formation of a tough coating of Al2O3 which sticks tightly to the bulk Al metal and protects it. This can be enhanced by anodising the metal. The metal is made the anode in an electrolytical cell and oxidised to form the protective film of the oxide. Nitric acid can be used to passivate some metals such as stainless steels. Some couples have very positive potentials but the species can still exist is water. Examples include: O2|H2O pH 7 Cr2O72-|Cr3+ Eo = 1.38 V 0.82 V 2+ o MnO4 |Mn E = 1.51 V Hence at pH 7 the Ecell for oxidation of water to produce O2 is 0.56 V and 0.69 V, respectively. H+|H2 Reason why Cr2O72- and MnO4- can still exist: Requires a 3e and a 5e transfer, respectively. Multiple electron transfer reactions are usually very slow. These species are under kinetic control -0.41 V Natural waters potential controlled by amount of dissolved O2 and by the presence of reducing couples from organic matter Fig.5.12 pH controlled by CO2/H2CO3/HCO3-/CO32- equilibrium Disproportionation reactions Since Cu+|Cu Cu2+|Cu+ Eo = 0.52V Eo = 0.16 V Cu+ lies in the stability field of water But Cu+ solutions are unstable because they can undergo disproportionation 2Cu+ → Cu2+ + Cu(s) A disproportionation reaction is one where the oxidation state of an element is simultaneously increased and decreased Disproportionation occurs because: Cu+ + e → Cu Cu+ → Cu2+ + e Eo = 0.52 Eo = -0.16 2Cu+ → Cu + Cu2+ Eo = 0.36 V In a comproportionation reaction two species of an element in two different oxidation states form a product in which the element is in an intermediate oxidation state Ag2+ + Ag → 2Ag+ Eo = 1.18 V Representing potential data Latimer diagrams summarise the standard potential (in V) between species of an element. +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 in acid So we can immediately write down the half reaction and the potential connecting any two species. For example, ClO3- and HClO2 ClO3- HClO2 - Balance O by adding H2O ClO3- HClO2 + H2O - In acid, balance H by adding H+. (In base, add H2O to the side of the equation requiring H, and OH- to the other side.) ClO3- + 3H+ HClO2 + H2O - Balance charges by adding e ClO3- + 3H+ + 2e HClO2 + H2O Eo = +1.18V +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 in acid Suppose we are interested in the potential between two non-adjacent couples. Goverall = Gindividual steps (First Law of Thermodynamics) -noverall FEooverall = -n1FEo1 + -n2FEo2 + -n3FEo3 +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 in acid o + -n FEo o -n FE + -n FE 1 1 2 2 3 3 Eooverall = -noverallF + n3Eo3 = n1Eo1 + n2Eo2 n1 + n2 + n3 = 2 x 1.65 + 1 x 1.67 2+1+1 = 1.65 V + 1 x 1.36 +1.20 ClO4+7 +1.18 +1.65 ClO3- HClO2 +5 +3 +1.67 HClO +1 +1.36 Cl2 Cl- 0 -1 and this refers to the reaction HClO2 + 3H+ + 4e Cl- + 2H2O in acid Frost diagrams are plots of X(N)/X(0) (in units of nEo) against the oxidation number N (or n) X(N) + Ne- X(0) Since G = -nFEo, G -nEo Eo Example Construct a Frost diagram from the following Latimer diagram (in aq acidic solution) Tl3+ +1.25 Tl+ -0.34 +0.72 Tl+ + e = Tl0 Eo = -0.34 V n=1 nEo = -0.34 V Tl3+ + 3e = Tl0 Eo = +0.72 V n=3 nEo = +2.16 V Tl Tl+ + e = Tl0 Eo = -0.34 V n=1 nEo = -0.34 V Tl3+ + 3e = Tl0 Eo = +0.72 V n=3 nEo = +2.16 V 3 nEo 2 +2.16 V 1 0 -0.34 V -1 0 1 2 3 4 N Note that in general n2Eo2 nEo n2Eo2 – n1Eo1 Eo = n2 – n1 n1Eo1 n2 n1 N Tl+ is the most stable oxidation state Tl3+ is a strong oxidising agent because slopes of the lines to the other two states are large and positive See SA pp. 155-158 Pourbaix diagrams Plots of E vs pH We will, as an example, derive the Pourbaix diagram for iron Two Latimer diagrams pertain In acid ([H+] = 1 M): 0.77 V Fe3+ -0.44 V Fe(OH)2 Fe In alkali ([OH-] = 1 M) -0.56 V Fe3+ -0.887 V Fe(OH)2 Fe Pourbaix diagrams: •correlate Latimer diagrams at pH 0 and pH 14 •take into account speciation or oxidation state of the element 0.77 V Fe3+ -0.44 V Fe(OH)2 The half reaction Fe3+ + e → Fe2+ Eo = 0.77 V does not involve a proton so Eo is independent of pH Fe Fe3+ + e → Fe2+ Fe3+ will precipitate out of solution as pH is increased. We can calculate the pH at which this will occur from the KSP for Fe(OH)3. Fe(OH)3(s) Fe3+ + 3OH– KSP = 4.11 x 10-37 M4 At what pH will [Fe3+] = 1.00 M? KSP = 4.11 x 10-37 M4 = [Fe3+][OH–]3 [OH–] = (4.11 x 10-37/1)0.333 = 7.43 x 10-13 M So [H+] = 10-14/7.43 x 10-13 = 1.35 x 10-2 M hence pH = 1.87 Fe(OH)3 Fe3+ + 3OH- Vertical lines in a Pourbaix diagram indicate where two species of an element in the same oxidation state are in equilibrium To calculate the Fe(OH)3|Fe2+ line... Fe3+ + e → Fe2+ Eo = 0.77 V 3OH- + 3H+ → 3H2O Go = -74.3 kJ mol-1 -239.7 kJ mol-1 Fe(OH)3 → Fe3+ + 3OH- Fe(OH)3 + 3H+ + e → Fe2+ + 3H2O 207.6 kJ mol-1 Go = -nFEo -106.4 kJ mol-1 = -1 x 96485 x 0.77 Go = -RT ln KSP = -8.315 x 298 x ln (4.11 x 10-37) Go = -nFEo Eo = -Go /nF = 106400/1 x 96485 = 1.10 V Fe(OH)3 + 3H+ + e → Fe2+ + 3H2O Eo = 1.10 V E = Eo – RT/nF ln Q E = 1.10 – 3 x 0.0592 x pH This must cross the Fe3+/Fe(OH)3 line when 0.77 = 1.10 – 3(0.0592)pH or pH = 1.87 which confirms the result we got from the KSP calclation 0.77 V Fe3+ -0.44 V Fe(OH)2 Fe 1.1 Fe(OH)3 + 3H+ + e → Fe2+ + 3H2O 1.1 Fe(OH)3 + 3H+ + e → Fe2+ + 3H2O From the KSP for Fe(OH)2 Fe(OH)2 Fe2+ + 2OH– KSP = 1.61 x 10-15 M3 At what pH will [Fe2+] = 1.00 M? KSP = 1.61 x 10-15 M3 = [Fe2+][OH–]2 [OH–] = (1.61 x 10-15/1)0.5 = 4.01 x 10-8 M So [H+] = 10-14/4.01 x 10-8 = 2.49 x 10-7 M hence pH = 6.61 1.1 Fe(OH)2 Fe2+ + 2OH- The half reaction Fe2+ + 2e → Fe Eo = -0.44 V does not involve a proton so Eo is independent of pH 1.1 Fe2+ + 2e → Fe An expression for the potential for the Fe(OH)3|Fe(OH)2 couple can be derived from the following data Fe(OH)3 + 3H+ + e → Fe2+ + 2H2O Eo 1.10 V Go -106.4 kJ mol-1 3H2O → 3H+ + 3OH- 239.7 kJ mol-1 Fe2+ + 2OH- → Fe(OH)2 -84.4 kJ mol-1 Fe(OH)3 + e → Fe(OH)2 + OH- Eo -0.51 V Go 48.9 kJ mol-1 E = Eo – RT/nF ln Q E = -0.51 + 0.0592 x pOH E = -0.51 + 0.0592 x (14 – pH) E = 0.316 – 0.0592 x pH 1.1 0.316 Fe(OH)3 + e → Fe(OH)2 + OH- ...and finally the value of Fe(OH)2|Fe couple can be found by similar considerations, and the Nernst equation applied. E = -0.060 – 0.0592 x pH Overlaying Pourbaix diagrams The feasibility of a reaction can be predicted by overlaying the relevant Pourbaix diagrams stability field for As(V) stability field for As(III) At pH < 5.5 and at pH > 9, Fe3+ has the potential to oxidise As3+ to As5+ 5.5 9 For example 0.65 0.45 Fe(OH)3 + e + 3H+ → Fe2+ + 3H2O E = 0.65 2 As3+ → As5+ + 2e E = -0.45 As3+ + 2Fe(OH)3 + 6H+ → 2Fe2+ + 6H2O + As5+ E = 0.20 V For 5.5 < pH < 9 As5+ will oxidise Fe2+ to Fe3+ The effect of complex formation on Eo values The Eo value of a metal ion is very dependent on the ligands of the ion Example, for the Fe3+|Fe2+ couple Ligand phenanthroline H 2O CN- Eo /V 1.14 0.77 0.36 Ligand phenanthroline H2O CN- N N N N N N N Fe Fe N N N back bonding from metal to phen ligand stabilises Fe(II) Eo /V 1.14 0.77 0.36 Ligand phenanthroline H2O CN- Eo /V 1.14 0.77 0.36 - CN - - NC CN Fe - - NC CN - CN Negatively charged ligands favour the higher positive charge of Fe(III) NH3 H 3N Co3+|Co2+ NH3 Co H 3N 0.11 V NH3 NH3 is a better donor ligand than H2O and so stablises Co(III) NH3 OH2 H2 O OH2 1.84 V Co H 2O OH2 OH2