Respiratory Alkalosis
advertisement

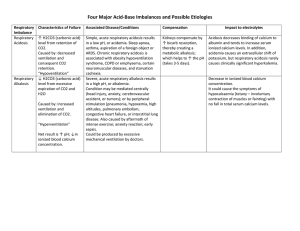
Acid –Base balance • An acid is any substance capable of liberating a hydrogen ion. An acid can be strong or weak, depending on the degree to which it breaks down to liberate hydrogen ions. For example, hydrogen chloride (HCl) rapidly and totally breaks down into hydrogen and chloride ion; therefore, it is considered a strong acid. In contrast, few lactic acid molecules break down to hydrogen ion and lactate; therefore, lactic acid is considered a weak acid. • A base is any substance that can accept a hydrogen ion, thereby taking it out of solution. A base can be strong or weak, depending on the degree to which it accepts a hydrogen ion . Most acids and bases found in the body are weak. Buffers A buffer is a substance that can either take free hydrogen ions from a solution or release hydrogen ions to a solution, thereby preventing large fluctuations in pH. Weak acids and weak bases make good buffers. There are three important buffer systems in the body: 1-Bicarbonate-Carbonic Acid Buffer System This is the main buffer system in the body. When free hydrogen ions are added to blood containing bicarbonate, the bicarbonate ions bind with the hydrogen ions, becoming carbonic acid (H2CO3) thereby preventing a significant decrease in blood pH. Carbonic acid is considered a weak acid; bicarbonate ion is considered its weak, conjugate (complementary) base. Carbonic acid can also dissociate to form carbon • dioxide and water; therefore, the bicarbonate buffer system is primarily used to eliminate hydrogen ion from the body through the elimination of the volatile gas carbon dioxide. The breakdown of carbonic acid to carbon dioxide and water requires the enzyme carbonic anhydrase, which is present in red blood cells. The reaction of carbonic acid to carbon dioxide and water is reversible, and carbon dioxide and water can rejoin to form carbonic acid. 2-Phosphate Buffer System Phosphoric acid (H2PO4-) is a weak acid; it dissociates in plasma to phosphate (HPO42-) and hydrogen ion (H+). Phosphate is a weak base. The kidney uses this system to buffer the urine as it excretes hydrogen ion.. 3-Hemoglobin Buffer System The third buffer system is provided by proteins in the blood, especially hemoglobin present in red blood cells. Hemoglobin binds to free hydrogen ions so blood pH decreases .As the blood flows through the lungs, hydrogen ions dissociate from the hemoglobin and join with bicarbonate to become carbonic acid which breaks down to carbon dioxide and water. Respiratory Control of Acid-Base Balance The lungs rid the body of carbon dioxide. Although carbon dioxide itself is not an acid, it joins with water to form carbonic acid . The minute-by-minute regulation of plasma pH is controlled by an increase or decrease in the rate of respiration. This system is possible because of the sensitivity of the respiratory center in the brain to free hydrogen ions which usually vary in accordance with carbon dioxide. Renal Control of Acid-Base Balance(Urine Buffers) Non-volatile acids(as lactic acid, acetic acid, acetoacetic acid) produced during metabolism are excreted in the urine. Their excretion occurs as a result of active secretion of hydrogen ions by cells of the kidney into the urine filtrate. In the filtrate, hydrogen ions join with phosphate, sulfate, or ammonia (NH3) buffers and are then excreted in the urine as salts of phosphoric acid, sulfuric acid, or ammonium ion (NH4+). Normal values: pH : 7.35-7.45 PaO2 : 80-100 mmHg PaCO2: 35-45mmHg HCO3 : 22-26mEq/ Acidemia The decrease in arterial pH to less than 7.35 is called acidemia. Acidemia may result from respiratory, renal, or metabolic causes. Acidosis *Is a systemic increase in hydrogen ion concentration. *Causes : -failure of the lungs to eliminate Co2 -excess production of volatile or non-volatile acids. -loss of bicarbonate base caused by persistent diarrhea -failure of the kidney either to reabsorb bicarbonate or to secrete hydrogen ions. Alkalemia Alkalemia is the increase in arterial blood pH above 7.45. Alkalemia may result from respiratory, renal, or metabolic causes. Alkalosis Alkalosis is a systemic decrease in hydrogen ion concentration Causes : -excess loss of carbon dioxide during hyperventilation. -excess loss of non-volatile acids during vomiting. - excess ingestion of a base. Compensation • If acidosis or alkalosis results from a metabolic or a renal disorder, the respiratory system responds by increasing or decreasing respiratory rate, this is called respiratory compensation. It occurs immediately upon changes in hydrogen ion concentration, because H+ controls respiratory center . • If acidosis or alkalosis results from a respiratory disorder, the kidneys respond by altering their handling of hydrogen ion and bicarbonate base to return the pH back toward normal, this is called renal compensation. Renal compensation begins to have an effect approximately 24 hours after a respiratory alteration in pH. Despite this delay, renal compensation is powerful. Respiratory Acidosis Is the decrease in arterial pH resulting from a primary respiratory disorder. If respiration is impaired ,carbon dioxide levels increase, leading to increase in H+ concentration which if not buffered causes pH to decrease. Causes of Respiratory Acidosis - all obstructive pulmonary disorders (chronic obstructive lung disease, asthma). - hypoventilation of any origin, including drug overdose or airway obstruction. - severe pulmonary congestion . - reduced pulmonary blood flow . Compensation for Respiratory Acidosis When acidosis is caused by a respiratory problem, renal compensation occurs. Renal compensation results in : - increasing its secretion and excretion of acid - increasing its reabsorption of base. *Renal compensation takes at least 24 hours to begin. - Clinical Manifestations -Neurologic symptoms such as headache, behavioral changes, and tremors. -Respiratory depression from increased carbon dioxide may occur. Diagnostic Tools -The partial pressure of carbon dioxide is greater than 45 mmHg . - Plasma bicarbonate levels are increased (greater than 28 mEq/L), reflecting the fact that the kidney is excreting more hydrogen ion and reabsorbing more base. -Urine pH is acidic as the kidneys excrete more hydrogen ion. N.B. If renal compensation is successful, plasma pH will be low, but in normal range Complications Paralysis and coma may result from cerebral vasodilatation in response to increased carbon dioxide concentration if levels become toxic. Treatment Improvement of ventilation is essential. Mechanical ventilation may be required. Respiratory Alkalosis The increase in arterial pH resulting from any primary respiratory disorder is called respiratory alkalosis. Respiratory alkalosis results when carbon dioxide partial pressure levels decrease to less than 35 mmHg. With decreased carbon dioxide, equation Co2+H2O ---H2CO3- -- H+--- +HCO3- is driven to the left, resulting in a decrease in free hydrogen ion concentration and an increase in pH. Causes of Respiratory Alkalosis -Hyperventilation (fever and anxiety). -Hypoxemia if the partial pressure of oxygen in arterial blood decreases to less than 50 mmHg (normal is approximately 100 mmHg). - Salicylate toxicity & brain infections can directly stimulate the respiratory center . Compensation for Respiratory Alkalosis Renal compensation involves decreasing its secretion and excretion of hydrogen ion and actively secreting bicarbonate ion into the urine. Again, this requires 24 hours to become effective. Clinical Manifestations - Rapid respirations. - Neurologic disturbances as dizziness, muscle contractions, and changes in consciousness. Diagnostic Tools -Decreased partial pressure of carbon dioxide of less than 35 mmHg . -For respiratory alkalosis lasting longer than 24 hours, bicarbonate blood levels are decreased (less than 22 mEq/L), reflecting the fact that the kidney is reabsorbing less base or secreting base into the urine. -Urine pH is basic as the kidneys attempt to excrete more bicarbonate base and return pH toward normal. Complications : -Convulsions and coma if the condition persists or becomes very severe. Treatment -Determining and treating the cause of hyperventilation is the most successful therapy. -Increasing partial pressure of carbon dioxide by breathing into a bag and rebreathing the expired air may reverse the alkalosis in an acute situation. Metabolic Acidosis The decrease in arterial pH resulting from a nonrespiratory problem is called metabolic acidosis. Metabolic acidosis is characterized by the accumulation of non-volatile acids. Causes of Metabolic Acidosis 1-Increase in non-volatile Acids caused by: -prolonged hypoxia(lactic acid) - fat metabolism in diabetics(ketones) -overdose of drugs such as salicylates . -excess protein metabolism ( starvation or malnutrition) 2-Decrease in the Renal Clearance of Hydrogen Ion caused by: - renal failure - interruption in renal blood flow. As a result of these conditions, the kidney, which normally reabsorbs all filtered bicarbonate and actively secretes hydrogen ion, cannot function properly, causing hydrogen ion to accumulate. 3-Loss of Bicarbonate caused by: - decreased renal function( loss of bicarbonate occurs)leads to acidosis. - chronic diarrhea (bicarbonate is concentrated in intestinal secretions). - high levels of extracellular chloride (hyperchloremia) cause metabolic acidosis because bicarbonate ions shift intracellularly( hyperchloremic acidosis). Compensation for Metabolic Acidosis - Respiratory compensation which involves increases in the rate and depth of respirations.It begins almost immediately with the onset of acidosis. * success depends on the severity of the acidosis. - Renal compensation(if healthy) by excreting more acid. Clinical Manifestations -weakness and fatigue occurring from poor muscle function. -Anorexia, nausea, and vomiting. -Warm flushed skin resulting from decrease in vascular response to sympathetic stimuli. *If metabolic acidosis is caused by diabetic ketoacidosis, additional manifestations will include: - Ketone smell (fruity) on breath - Anorexia, nausea and vomiting, abdominal pain - Increased rate and depth of respirations - Decreasing level of consciousness, leading to coma *If metabolic acidosis is caused by diarrhea, additional manifestations will include: - Signs of dehydration, including decreased blood pressure and loss of skin turgor - Abdominal pain and cramping - Frequent, loose stools Diagnostic Tools - decreased bicarbonate levels to less than 22 mEq/L . - Partial pressure of carbon dioxide is less than 35 mmHg(rapid deep respiration ). * If respiratory compensation is successful, pH is low but in the normal range. * If compensation is unsuccessful, plasma pH will reflect high plasma acidity and be less than 7.35, even in the face of reduced carbon dioxide. - Urine pH will be acidic if renal function is normal, because the kidneys will attempt to excrete more acid to return pH toward normal. * If metabolic acidosis is caused by diabetic ketoacidosis, additional diagnostic tools will include: -Increase in blood and urine glucose -Ketonuria and decreased urine pH * If metabolic acidosis is caused by chronic renal failure, additional diagnostic tools will include: -Urine pH only slightly acidic or non-acidic - Increased blood urea nitrogen (BUN), reflecting excess protein catabolism (breakdown) and decreased GFR Complications *If metabolic acidosis is caused by chronic renal failure, complications may include renal osteodystrophy (bone dissolution due to renal disease) and renal encephalopathy. *If pH falls below 7.0, cardiac dysrhythmia can occur. This happens as a result of changes in cardiac conduction, which occur in direct response to a decrease in pH and because of the effects of increased hydrogen ion concentration on plasma and intracellular potassium. Treatment -treating the cause. -for patients with renal disease, excess base in the diet is given. -Administration of sodium bicarbonate infusion may be used to raise pH rapidly if the person is at risk of dying. This procedure must be undertaken carefully because it may cause brain swelling. The increase in arterial pH resulting from a nonrespiratory problem is called metabolic alkalosis. Causes of Metabolic Alkalosis - excessive loss of acid - increased base ingestion - dehydration ( can lead to less bicarbonate to be filtered across the glomerulus). - excessive vomiting (chloride or potassium deficiency) - ingestion of bicarbonate to treat indigestion or heartburn Compensation for Metabolic Alkalosis Respiratory compensation ( decrease in the rate and depth). It can occur almost immediately upon onset of alkalosis. The kidneys will also participate in compensation when possible. Clinical Manifestations -Neurologic manifestations are slow to develop, but may include confusion, hyperactive reflexes, spasms, and tetany (sustained muscle contraction). Diagnostic Tools -Increased bicarbonate levels greater than 28 mEq/L (because increased bicarbonate either is the direct cause of the alkalosis or reflects a decrease in hydrogen ion concentration). -Because of respiratory compensation, carbon dioxide levels is greater than 45 mmHg. The rate and depth of respirations are reduced, possibly causing hypoxemia. - -If respiratory compensation is successful, plasma pH will be high but in the normal range. If compensation is unsuccessful, plasma pH will reflect the high plasma base concentration and will be greater than 7.45 in the face of elevated carbon dioxide. -Urine pH will be basic if renal function is normal, because the kidneys will attempt to excrete less acid and more base to return pH toward normal. Complications With pH greater than 7.55, dysrhythmia and coma may result from alterations in neuronal and cardiac muscle cell depolarization. Treatment -If the cause is due to chloride or potassium deficiency, these ions must be replaced. -If decreased extracellular volume is the cause, saline solution replacement is required Summary *Note, if the pH is low (acidosis),if high (alkalosis) *if PaCO2 is outside the normal range, the problem is respiratory *if HCO3 is outside the normal range, the problem is metabolic • • • • • • • • • • • • • • • • • • • • Respiratory acidosis: Ph = 7.32 PaCO2 = 67 PaO2 = 47 HCO3 = 37 *Respiratory alkalosis: Ph = 7.6 PaCO2 = 30 PaO2 = 60 HCO3 = 22 *Metabolic acidosis: Ph = 7.18 PaCO2 = 38 PaO2 = 70 HCO3 = 15 *Metabolic alkalosis: Ph = 7.58 PaCO2 = 35 PaO2 = 75 HCO3 = 50