2.1 Molecules to Metabolism - NOTES
advertisement

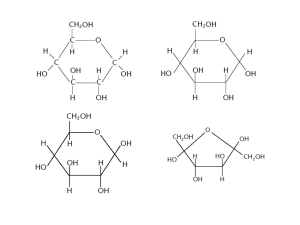
2.1 Molecules to Metabolism Biology Journal 8/25/2014 Complete the table! Molecule Type Monomer Dimer Polymer Examples Carbohydrates Proteins Lipids (aka fats) Starch… Enzymes, collagen, elastin, keratin Monopeptide (one amino acid) CH2 n/a lipid Biology Journal 8/25/2014 Molecule Type Monomer Dimer Polymer Carbohydrates Monosaccharide Disaccharide Polysaccharide Monopeptide Proteins (one amino acid) Dipeptide Polypeptide, Protein Lipids (aka fats) CH2 n/a lipid Examples Starch, lactose, glucose, ribose Enzymes, keratin, elastin, Fatty acids, triglyceride Biology Journal 8/25/2014 1. What kind of reaction is shown? 2. Write out the reaction as words: Sucrose + _______ → ________ + _______ 3. When might this reaction be happing in your life? + H2 O → + Biology Journal 8/25/2014 1. 2. 3. What kind of reaction is shown? Catabolism (breaking down) through hydrolysis Write out the reaction as words: see below When might this reaction be happing in your life? This happens whenever you eat sucrose (sugar)! + sucrose H2 O → + water → + fructose + glucose We will now learn how the digestive system makes this happen! Biology Journal 8/27/2013 What is the name of the monosaccharides shown below? These 2 molecules bond in a condensation reaction to form a disaccharide called lactose. Show this reaction by circling the atoms that are “released” as water, and draw the product. Biology Journal 8/27/2013 What is the name of the monosaccharide below? These 2 molecules bond in a condensation reaction to form a disaccharide called lactose. Show this reaction by circling the atoms that are “released” as water, and draw the product. + → + H2O Biology Journal 8/19/2014 What is a monomer? What is a dimer? What is a polymer? glucose lactose starch Biology Journal 8/20/2014 Label each drawing with its name. Annotate the pictures with a description of the elements found in them, and their relative amounts. (you don’t have to draw the pictures in your notebook, just write the names and describe them) Fatty acid Has 2 O’s (carboxyl) Long chain of C’s and H’s Glucose General formula = CnH2nOn Amino acid Complex C and H structure Has O’s (carboxyl) Has N (amino) R group can be many things Fatty acid (again!) Ribose General formula = CnH2nOn Biology Journal 8/21/2014 A chemical reaction occurs, synthesizing 5 glucose molecules. a. What is the name of this chemical reaction? b. What is the name of the product? c. What is the other product and how many molecules of it are made? H OH H2 O H OH H2 O OH H H2 O (a) Condensation anabolism (building up) (b) starch or cellulose (c) 4 H2O molecules are made H OH H2 O Topic 2: Molecular biology (21 hours) 2.1 Molecules to metabolism: Living organisms control their composition by a complex web of chemical reactions. Nature of science: Falsification of theories—the artificial synthesis of urea helped to falsify vitalism. Understandings: Molecular biology explains living processes in terms of the chemical substances involved. Carbon atoms can form four covalent bonds allowing a diversity of stable compounds to exist. Life is based on carbon compounds including carbohydrates, lipids, proteins and nucleic acids. Metabolism is the web of all the enzymecatalysed reactions in a cell or organism. Anabolism is the synthesis of complex molecules from simpler molecules including the formation of macromolecules from monomers by condensation reactions. Catabolism is the breakdown of complex molecules into simpler molecules including the hydrolysis of macromolecules into monomers. Applications and skills: Application: Urea as an example of a compound that is produced by living organisms but can also be artificially synthesized. Skill: Drawing molecular diagrams of (alpha-D and beta-D) glucose, D-ribose, a saturated fatty acid and a generalized amino acid (using R). Skill: Identification of biochemicals such as sugars (mono- and di-saccharides), lipids (triglycerides and phospholipids, saturated and unsaturated) or amino acids (R groups do not need to be memorized) from molecular diagrams. Skill: Food tests such as the use of iodine to identify starch or Benedict’s reagent to identify reducing sugars could be carried out. Biology Journal 8/18/2014 2.1 Homework Quiz! 1. What are the 4 most common elements found in living things? (you can use their atomic symbols to answer) 2. Glucose and cellulose are examples of: a. Carbohydrates b. Lipids c. Proteins d. Nucleic acids 3. True or false: In the process of anabolism, polymers are broken down into their component monomers. 4. What are the names of any 2 nucleic acids? 5. Give the names of 2 common examples of proteins. All of these represent the same substance: glucose. There are many ways of expressing the idea of a molecule. C6H12O6 Chemical Formula Lewis-stick drawing Spacefilling model Skeletal-line Drawing Ball and Stick model Skeletal Line Drawings are often used when representing large, complex biological molecules. 1. 2. 3. 4. Rules for Skeletal Line Drawings: Lines are covalent bonds C’s are “corners” H’s attached to C’s are not drawn (remember: C makes 4 bonds) Other elements, and H’s attached to them, are shown with their symbol This is nicotine. What’s its chemical formula? H C10H14N2 H C HC C C H C CH C H2 CH2 C H2 Rules for Skeletal Line Drawings: 1. 2. 3. 4. Lines are covalent bonds C’s are “corners” H’s attached to C’s are not drawn (remember: C makes 4 bonds) Other elements, and H’s attached to them, are shown with their symbol This is Cocaine. It can be turned into crack. What’s its chemical formula? C10H14N2 H C C H C C C C C H C H H H C H2 Rules for Skeletal Line Drawings: 1. 2. 3. 4. Lines are covalent bonds C’s are “corners” H’s attached to C’s are not drawn (remember: C makes 4 bonds) Other elements, and H’s attached to them, are shown with their symbol This is adrenaline, which is stored in your adrenal glands, and released as part of the “fight or flight response.” C C H C C H C C H C H2 C H C9H13NO3 C H3 These are the same molecule, called a fatty acid. They are represented in different ways. All of these represent the same substance: glucose. There are many ways of expressing the idea of a molecule. C6H12O6 Chemical Formula Lewis-stick drawing Spacefilling model Skeletal-line Drawing Ball and Stick model The process of science is based on a system of: • Making hypotheses • Testing them through rigorous collection of empirical evidence (can be measured and recorded) • Peer-review of data • Statistical analysis • Replication • Acceptance THEORY! Highest level of certainty in science acceptance corroborated unquestionably repeated corroborated ? observations hypothesis hypothesis ? “It’s only a theory” testing not corroborated (evidence does not support hypothesis) The scientific process Falsifiability • The purpose of science is to explain and predict. • Falsifiability is the possibility that an statement could be proven false. • This does not mean it is false; rather, it means that if the statement were false, then its falsehood could be demonstrated. No human lives forever. Is this falsifiable? No. • It does not seem possible to prove wrong. • In theory, one would have to observe a human living forever to falsify that claim. All humans live forever. Is this falsifiable? Falsifiable. • Observing just one dead human could prove the statement wrong. • A hypothesis, proposition, or theory is "scientific" only if it is, among other things, falsifiable. • Unfalsifiable statements are non-scientific. The theory that the molecules of living things came only from a special “vital principal” was proven false, when urea was synthesized artificially. Urea was discovered in the 1720’s in urine. In 1828 Friedrich Wohler synthesized urea! Silver isocyanate It was assumed to be a product of the kidneys. At the time, it was thought that these compounds could only be made with the help of a “vital principle”. Ammonium chloride Yea, I like pee. So what?! This “vital principle” causes the phenomena of life to happen, rather than chemical or physical forces. If urea had been synthesized without a “vital principle” then other organic compounds could as well. Molecules can be represented in lots of different ways. Look at the following diagrams, they all represent the same thing. How? Molecules can be represented in lots of different ways. Look at the following diagrams, they all represent the same thing. How? 2.1 Draw a molecular diagram of alpha-D glucose, D-ribose, a saturated fatty acid, and a generalized amino acid (showing R group) Glucose C6H12O6 2.1 Draw a molecular diagram of alpha-D glucose, D-ribose, a saturated fatty acid, and a generalized amino acid (showing R group) Ribose C5H10O5 We put the D in DNA DNA is deoxyribonucleic acid. A form of ribose with one fewer oxygen called “deoxyribose” is part of DNA’s structure. 2.1 Draw a molecular diagram of alpha-D glucose, D-ribose, a saturated fatty acid, and a generalized amino acid (showing R group) Saturated Fatty Acid 2.1 Draw a molecular diagram of alpha-D glucose, D-ribose, a saturated fatty acid, and a generalized amino acid (showing R group) Generalized Amino Acid This is an an amino acid. (this one is called asparagine) R group (in this case CH2CONH2) Amine (NH2) Carboxyl (COOH) Alpha carbon (the middle C where the R group is attached) Drawing structures Glucose – C6H12O6 • 6-membered ring with a side chain • 5 carbons in the ring, one carbon in the side chain • #C’s with 1 on the right, then clockwise • Hydroxyl’s (-OH) on C’s 1, 2, 3, 4 point down, down, up, down Ribose – C5H10O5 • 5-membered ring with side chain • 4 carbons in the ring, one carbon in the side chain • #C’s with 1 on the right, then clockwise • Hydroxyl’s (-OH) on C’s 1, 2, 3, point up, down, down Generalized amino acid • C atom in the center is bonded to four things • Amine group –NH2 • Carboxyl group – COOH • Hydrogen atom –H • R group Saturated fatty acid • C’s form an unbranched chain • All single bonds in a saturated fatty acid • # of C’s is between 14 and 20 • At one end of the chain, the C is part of a carboxyl group • At the other end of the chain, the C is bonded to three H atoms • All other C’s are bonded to two H atoms Lipids (aka fats) have repeating CH2 units. This gives them lots in energy (calories) in the numerous C-H bonds. • Most mammals (humans included), store their extra energy as the lipid triglyceride, shown below. Saturated fats have the maximum number of H’s. • • They are crammed, or “saturated” with H’s Thus, they have the most calories Animal fats are usually saturated. Unsaturated fats have one (monounsaturated) or more (polyunsaturated) double bonds. • • With double bonds, there is fewer room for H’s Thus, they have fewer calories, and are generally “healthier.” Plant fats are usually unsaturated. What is condensation? Condensation is the formation of water Model how fatty acids and glycerol combine to form triglycerides through condensation reactions OH + H = Build Triglyceride through a condensation reaction! 1. Cut out the molecules 2. Label each molecule with a marker 3. “Chop” off the H and OH from the appropriate molecules, and attach them together. 4. Put the H and OH on a water drop. It’s H2O! Attach it to the molecule nearby, to show that water was created through this process. Carbohydrates and Sugars • • • • General formula of CnH2nOn Monomer = sugar or monosaccharide Polymer = carbohydrate or polysaccharide Plants often store their extra energy as carbohydrates, rather than lipids. Disaccharides Sucrose = glucose + fructose Monosaccharides Lactose = glucose + galactose Glucose Polysacchardies Starch = glucose + glucose +glucose + glucose… Ribose Galatose Model how monosaccharides combine to form disaccharides through condensation reactions OH + H = Build Maltose through a condensation reaction! 1. Cut out the molecules 2. Label each molecule with a marker 3. “Chop” off the H and OH from the appropriate molecules, and attach them together. 4. Put the H and OH on a water drop. It’s H2O! Attach it to the molecule nearby, to show that water was created through this process. Build Starch through a condensation reaction! 1. Cut out the molecules 2. Label each molecule with a marker 3. “Chop” off the H and OH from the appropriate molecules, and attach them together. 4. Put the H and OH on a water drop. It’s H2O! Attach it to the molecule nearby, to show that water was created through this process. 5. Make a starch polymer, consisting of 4 monomers! Amino Acids • Have amine group (NH2), carboxyl group (COOH), alpha carbon, and side chain (R). • Make polymers called polypeptides, or proteins. Tyrosine Serine Glutamine There are 20 (or more, depending on how you count) amino acids used in the human body. Anabolism = building a polymer from monomers. Makes H2O (condensation) Ex: your body making hair Catabolism = Breaking down a polymer into monomers. Consumes H2O (hydrolysis). Ex: digesting a muffin Build a Protein through condensation anabolism! 1. A maximum of 4 students in a group. – (more than 4 names on a model will result in ½ credit) 2. Look at your sequence and ID the amino acids you need. Label each amino acid with a marker. 3. “Chop” off the H and OH from the appropriate molecules, and attach them together, show 1 molecule “drop” of H2O is made and attach it nearby. 4. Bond all of the amino acids in the correct sequence. Label what sequence number you have is on your model! 2.1 Skill: Food tests such as the use of iodine to identify starch or Benedict’s reagent to identify reducing sugars could be carried out. An Iodine Solution (yep, the element) can be used to identify if starch is present. Iodine Solution + Starch Solution → Positive Test Starch Test: https://www.youtube.com/watch?v=ebaQkkw2DCs 2.1 Skill: Food tests such as the use of iodine to identify starch or Benedict’s reagent to identify reducing sugars could be carried out. Benedict’s Reagent can be used to identify all monosaccharides, and many disaccharides. It must be mixed, then heated. Benedict’s Reagent Test: https://www.youtube.com/watch?v=Lt7RCIfudYQ Question from Paper 1 Which molecule represents ribose? What is molecule B? What is molecule C? Question from Paper 1 Which structure represents an amino acid? Question from Paper 1 Which molecule is: i. ribose ii. Generalized fatty acid ii. Generalized amino acid Discuss which two molecules are most similar in structure. a. What kind of molecule is this? b. Label its parts. R group (in this case CH2CONH2) Amine (NH2) Carboxyl (COOH) Alpha carbon a) amino acid (in this case asparagine) a. What is the name of this molecule? b. Label its parts Carboxyl groups Glycerol a. triglyceride 3 Fatty acids What kind of molecule is this? It’s a lipid! Specifically, a phospholipid. a. Name this molecule. b. Describe it’s structure in as much detail as you can. a. Fatty acid. b. It is a lipid, it has a carboxyl group (COOH), and it is unsaturated (it has a double bond), which makes it have fewer calories than a saturated version. 1. What kind of reaction is shown? 2. Write out the reaction as words: Sucrose + _______ → ________ + _______ 3. When might this reaction be happing in your life? + H2 O → + 1. 2. 3. What kind of reaction is shown? Catabolism (breaking down) through hydrolysis Write out the reaction as words: see below When might this reaction be happing in your life? This happens whenever you eat sucrose (sugar)! + sucrose H2 O → + water → + fructose + glucose A chemical reaction is shown below. 1. Name each reactant and product. 2. What kind of reaction is this? 3. Where should water be present in the reaction? How many molecules of water? → A chemical reaction is shown below. 1. Name each reactant and product. 2. What kind of reaction is this? 3. Where should water be present in the reaction? How many molecules of water? Catabolic Hydrolysis Reaction Monopeptide (amino acid) Dipeptide + H2O Monopeptide (amino acid) → • Hydrolysis (water is split) • It “fills in” each monomer A chemical reaction occurs, synthesizing 5 monomers. a. What could be two terms to describe this reaction? b. What is the name of the product? c. What is the other product and how many molecules of it are made? H OH H2 O H OH H2 O OH H H2 O (a) Condensation anabolism (building up) (b) starch or cellulose (c) 4 H2O molecules are made H OH H2 O