organic chem ppt notes
advertisement

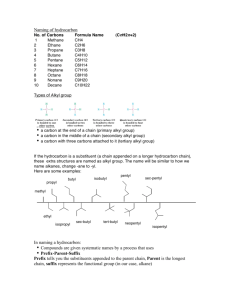
Organic Chemistry Essentials • Chemistry of carbon compounds • Carbon can form 4 covalent bonds with other atoms. This allows it to make millions of different compounds. • Carbon can form single, double, and triple bonds. Hydrocarbon • compound containing only hydrogen and carbon • Ex. CH4 C2H6 Hydrocarbon derivative • compound with some hydrogen atoms replaced by other elements (O, N, F, Cl, Br, I) • ex. CH3Br Straight chained hydrocarbon Ex. CH3CH2CH2CH2CH2CH3 or C6H14 Branched hydrocarbon CH3 CH3CH2CHCH2CH3 or C6H14 Saturated hydrocarbon • no double or triple bonds Unsaturated hydrocarbon • contains double and/or triple bonds • not “saturated” with hydrogen Structural formulas Condensed Structural formulas CH3-CH3 Alkanes • • • • hydrocarbons with only single bonds General formula: CnH2n+2 names end in -ane boiling pt. increases with # of carbons IUPAC Names Name # carbons Structural Formula Methane 1 CH4 Ethane 2 CH3CH3 Propane 3 CH3CH2CH3 Butane 4 CH3CH2CH2CH3 Pentane 5 CH3CH2CH2CH2CH3 Name # carbons Structural Formula Hexane 6 CH3CH2CH2CH2CH2CH3 Heptane 7 CH3CH2CH2CH2CH2CH2CH3 Octane 8 Nonane 9 CH3CH2CH2CH2CH2CH2CH2CH3 CH3 CH2 CH2CH2CH2CH2CH2CH2CH3 Decane 10 CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 Alkenes • contain at least one double bond • name ends in -ene • general formula: CnH2n Alkynes • contain at least one triple bond • name ends in -yne • general formula: CnH2n-2 Nomenclature • naming system • IUPAC (International Union of Pure and Applied Chemistry) Rules for naming alkanes 1. Name the longest carbon chain in the structure. If the chain is cyclic, include the prefix cyclo. 2. Name, in alphabetical order, the kinds of groups which are attached to the chain you named in step 1. Use the appropriate prefix, such as di-, tri-, tetra-, etc., to indicate how many of each group are present. 3. Number the carbon atoms in the longest chain consecutively from the end of the chain which allows the attached groups to have the lowest numbers possible. Assign to each group you named in step 2 the number(s) indicating its position(s) on the main chain. Rules for naming alkenes and alkynes 1. Name the longest carbon chain containing the carbon-carbon double or triple bond. The name of the longest chain ends in – ene for an alkene; in –yne for an alkyne. The position of the double or triple bond in the carbon chain is indicated by a number before the name of the chain. 2. Name the groups attached as in alkanes. 3. When assigning numbers to atoms in the chain, start numbering from the end of the chain closest to the double or triple bond. Naming branched chain alkanes • substituent- atom or group of atoms that takes the place of hydrogen on a hydrocarbon molecule • ex. C, O, N, S, P, Cl, F, I, Br Alkyl Group • hydrocarbon substituent • alkane with one H removed • drop the -ane ending and add -yl H H C CH3 methyl H H H H C C H H CH3CH2 ethyl Name the following: 3,3-diethylhexane 4-ethyl-4,6-dimethyl-5-propylnonane 6-ethyl-4,5-dipropylnonane Draw the following: 2,2,4-trimethylpentane Draw the following: 3-ethyl-2-hexene Draw the following: 4-bromo-2,2-dichloro-3,5diethyl-6-fluorooctane Properties of Hydrocarbons • nonpolar • not attracted to water Structural Isomers • compounds that have the same molecular formula but different molecular structures. • physical and chemical properties differ Butane CH3-CH2-CH2-CH3 2-methylpropane or isobutane CH3 CH3-CH-CH3 Geometric Isomers • Each of the carbons of the double bond must have at least one substituent. • Ex. CH3CH=CHCH3 has 2 geometric isomers because the double bond is not free to rotate. Bond angle (sp2) is 120o. cis-2-butene trans-2-butene Stereoisomers • Stereoisomerism is the arrangement of atoms in molecules whose connectivity remains the same but their arrangement in space is different in each isomer. • The two main types of stereoisomerism are: • cis-trans isomerism • optical isomerism Optical isomerism is a form of isomerism where the two different isomers are the same in every way except being nonsuperimposable mirror images of each other. Optical isomers are known as chiral molecules. A compound is chiral when it cannot be superimposed on its mirror image. The pair of mirror imaged non-superimposable compounds are known as enantiomers. Even though very similar still, different enantiomers of the same chiral drug can have very different pharmological effects, mainly because the proteins they bind to are also chiral. 4 different groups attached to the carbon The two enantiomers of bromochlorofluoromethane Cyclic hydrocarbons • compounds that contain a hydrocarbon ring • general formula is: CnH2n Cyclopropane Cyclobutane Aliphatic compounds • hydrocarbon compounds which do not contain rings Arenes • unsaturated cyclic hydrocarbons • Benzene, C6H6, is an arene. • Benzene is a flat molecule with resonance. Shorthand ways to draw benzene: Aromatic compound • any substance in which the bonding is like benzene • A benzene ring used as a substituent on a hydrocarbon chain is called a phenyl group. • Ex. methylbenzene (toluene) Nomenclature: Natural gas- 80% methane, 10% ethane, 4% propane, 2 % butane Petroleum = complex mixture of hydrocarbons Cracking= a controlled process by which hydrocarbons are broken down or rearranged into smaller, more useful molecules Functional Group • Chemically reactive part of an organic molecule • Learn these functional groups • R means the rest of the molecule (usually hydrocarbon The Common Functional Groups Class Halohydrocarbons Alcohols Ethers Aldehydes General Formula RX ROH ROR O R C H The Common Functional Groups Class Ketones Carboxylic Acids Esters Amines General Formula O R C R' O R C OH O R C O R' RNH2 Halocarbons • Organic compounds containing F,Cl, Br, or I • Halogen groups are named as substituents just as alkyl groups are. chloromethane Cl I I Cl-C-Cl CH3-CH-CH-CH3 bromobenzene 2,3-diiodobutane H Cl-CH2-CH3CH3 Trichloromethane (chloroform) 1-chloropropane Alcohols Organic compounds with a hydroxyl group (-OH) Naming: drop “e”, add “-ol” Methanol Ethanol Methyl alcohol Ethyl alcohol “wood alcohol” “drinking alcohol” propanol propyl alcohol n-propanol 1-propanol 2-propanol isopropanol isopropyl alcohol “rubbing alcohol” Alcohols can be classified as primary, secondary or tertiary. antifreeze Glycerine 1,2,3-propantriol Phenol Properties of Alcohols • Hydrogen bonding • Short-chained alcohols are soluble in water Fermentation- production of ethanol from sugars by the action of yeast or bacteria C6H12O6 2CH3CH2OH + 2CO2 Denatured Alcohol- ethanol with an added substance to make it toxic Ethers • Compounds in which oxygen is bonded to two carbon groups • R-O-R’ • Name the R groups in alphabetical order and add the word ether. ethyl methyl ether dimethyl ether ethyl phenyl ether diethyl ether 1st reliable general anesthetic, 1842 Aldehydes • Organic compounds in which the carbon of the carbonyl group ( C=O ) is always joined to at least one H. • Naming: drop “e”, add “-al”, carbonyl carbon is #1 methanal (formaldehyde) ethanal CH3 H CH3CH2CHCH2C=O 3-methylpentanal Ketones • Organic compounds in which the carbon of the carbonyl group is joined to 2 other carbons. The carbonyl group is in the middle of the chain instead of the end of a chain as in an aldehyde. • Naming: drop “e”, and “one”. If carbonyl can be in more than one position, give it a # 3-hexanone Propanone (acetone) Naming: drop “e”, add “oic acid” The carboxyl carbon is always #1. butanoic acid methanoic acid “formic acid” propanoic Acid “propionic acid” Esters • Derivatives of carboxylic acids in which the –OH of the carboxyl group has been replaced by an –OR from an alcohol • Formed by combination of a carboxylic acid with an alcohol in a dehydration reaction • Often common flavors and odors Naming: Name alkyl group, then acid with –ate ending ethyl ethanoate or ethyl acetate (apple scent) methyl ethanoate methyl methanoate or methyl acetate or methyl formate Amines -NH2 • the suffix amine is added to the alkyl substituent • can also be named as an amino group 3-aminopropanoic acid ethylamine cyclohexylamine POLYMERS • made of repeating units called monomers