Lecture34
Stable Isotope
Geochemistry
Theory
Lecture 34
Beginnings
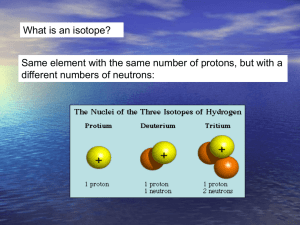
• Stable isotope geochemistry is concerned with variations in the isotopic compositions of elements arising small differences in the chemical behavior of different isotopes of an element
• These can provide a very large amount of useful information about chemical (both geochemical and biochemical) processes.
• Harold Urey essentially initiated this field, predicting that different isotopes would have slightly different behavior (the first success of which was the discovery of deuterium, for which he won the Nobel Prize).
• The story really begins in 1947 when he and colleagues predicted the oxygen isotopic composition of carbonates would vary with temperature.
• What has been learned in the 65 years since those papers were published would undoubtedly astonish even Urey.
• Urey was also instrumental in founding the modern fields of geochronology and cosmochemistry.
Harold Urey
Stable Isotopes
• Traditionally, the principal elements of interest in stable isotope geochemistry were H, C, N, O, and S.
• Over the last two decades, Li and B have also become “staples” of isotope geochemistry.
• These elements have several common characteristics: o They have low atomic mass.
o The relative mass difference between their isotopes is large.
o They form bonds with a high degree of covalent character.
o The elements exist in more than one oxidation state (C, N, and S), form a wide variety of compounds (O), or are important constituents of naturally occurring solids and fluids.
o The abundance of the rare isotope is sufficiently high (generally at least tenths of a percent) to facilitate analysis.
• t was once thought that elements not meeting these criteria would not show measurable variation in isotopic composition.
However, as new techniques have become available
(particularly MC-ICP-MS), geochemists have begun to explore isotopic variations of many more elements, including Mg, Si, Cl,
Ca, Ti, Cr, Fe, Zn, Cu, Ge, Se, Mo, Tl, and U, also isotopic variations are much smaller in these elements.
Delta Notation
• Stable isotope ratios variations are generally small, so they are reported as per mil (‰) deviations from a standard. For example, Oxygen isotope ratios
(except in carbonates) are reported relative to
Standard Mean Ocean Water (SMOW) as: d
18 O (‰)
=
é
é
(
18
O /
16
O
)
(
18 sample
O /
16
-
(
O
)
18
O /
SMOW
16
O
)
SMOW
é
é
é
é
1000
• Carbonates are reported relative to the Pee Dee
Belemite (PDB). They are related as:
δ 18 O
PDB
= 1.03086δ 18 O
SMOW
+ 30.86
Element
Hydrogen
Lithium
Boron
Carbon
Nitrogen
Oxygen
Sulfur
Common Stable Isotopes
Notation
δ
D
δ 7 Li
δ
11 B
δ
13 C
δ
15 N
δ
18 O
δ
17 O
δ
34 S
δ
33 S
Ratio Standard
D/H ( 2 H/ 1 H) SMOW
7 Li/ 6 Li
11 B/ 10 B
NIST 8545 (L-
SVEC)
NIST 951
13
15
C/ 12 C
N/ 14 N
Absolute ratio
1.557
12.285
× 10 -4
4.044
PDB 1.122 × 10 -2
Atmosphere 3.613 × 10 -3
18 O/ 16 O
17 O/ 16 O
34 S/ 32 S
33 S/ 32 S
SMOW, PDB 2.0052 × 10 -3
SMOW 3.76 × 10 -4
CDT
CDT
4.416 × 10 -2
7.877 × 10 -3
Fractionation Factor
• The fractionation factor, α, is the ratio of isotope ratios in two phases predicted or found to occur: a
A
-
B
º
R
A
R
B
• Fractionation factors can also be expressed as the difference in delta values:
∆
A-B
= δ
A
• The two are related as
- δ
B
∆ ≈ (α - 1) × 10 3 or ∆ ≈ 10 3 ln α
Fractionations
• Isotope fractionation can originate from both kinetic effects and equilibrium effects.
• Equilibrium Fractionations: o Quantum mechanics predicts that the mass of an atom affects its vibrational motion, and therefore the strength of chemical bonds. It also affects rotational and translational motions. From an understanding of these effects of atomic mass, it is possible to predict the small differences in the chemical properties of isotopes quite accurately.
• Kinetic Fractionations o Lighter isotopes form weaker bonds and therefore react faster. They also diffuse more rapidly. These also lead to isotopic differences.
Equilibrium Fractionations
• Equilibrium fractionations arise from translational, rotational and vibrational motions of molecules in gases and liquids and atoms in crystals.
• Isotopes will be distributed so as to minimize the vibrational, rotational, and translational energy of a system.
All these motions are quantized (but quantum steps in translation are very small).
• Of the three types of energies, vibrational energy makes by far the most important contribution to isotopic fractionation. Vibrational motion is the only mode of motion available to atoms in a solid.
• These effects are small. For example, the equilibrium constant for the reaction:
½C 16 O
H
2
+ H
2
16 O
2
18 O
⇄
½C 18 O
2
+
• is only about 1.04 at 25°C and the ∆G of the reaction, given by -RT ln K, is only
-100 J/mol
Predicting Fractionations
• Boltzmann distribution law states that the probability of a molecule having internal energy E i is:
P i
=
å n g i e
-
E i g n e
-
/k T
E i
/k T o g is a weighting factor to account for degenerate states.
• The denominator is the partition function:
Q
=
å
n g n e
-
E i
/k T
• From partition functions we can calculate the equilibrium constant:
K
=
Õ
i
Q i n i
•
• We can divide the partition function into three parts:
Q total
= Q trans
Q rot
Q vib o The separate partition functions can be calculated separately.
Partition Functions
• The following applies to diatomic molecules. Principles are the same for multiatomic molecules and crystals, but the equations are more complex because there are many possible vibrations and rotationns.
• Vibrational Partition Function: e
h n
/2 k T
Q vib
=
1
e
h n
/k T o where h is planks constant (converting frequency to energy) and ν is the vibrational frequency of the bond.
• Rotational Partition Function
Q rot
=
8 p
2
I k T s h
2 o where I is the moment of inertia I = µr 2 ; reduced mass of atoms times bond length.σ is a symmetry factor; σ= 1 for a non-symmetric molecule ( 18 O 16 O) and
2 for a symmetric one ( 16 O 16 O)
• Translational partition function (derived from
Schrödinger’s equation for particle in a box):
Q trans
=
(2 p m k T )
3/2
V h
3
Partition Function Ratios
• Since
K
=
Õ
Q i i
• what we really want is the ratios of partition functions for isotope exchange reactions. Most terms cancel (including bond length).
i n
• This ratio for two isotopic species (isotopologues) of the same diatomic molecules, e.g., H
2
18 O and H
2
16 O, will be:
Q
H
2
Q
H
2
18
O
16
O
= h s
H h n
18
2
H
O s
H n
16
2
H
O
18
2
O kT
16
2
O kT e
h n
H
18
O
/2 kT
1
e
h n e
h n
H
16
2
O
H
18
O
/2 kT
/ kT
1
e
h n
H
16
O
/ kT
µ
H
18
2
O m
H
3/2
18
2
O
µ
H
16
2
O m
H
3/2
16
2
O
= n n
H
18
2
O
H
16
2
O e
h n
H
2
O
/2 kT e
h n
H
16
2
O
/2 kT
(1
e
h n
H
2
O
/ kT
(1
e
h n
H
18
2
O
/ kT
)
µ
H
18
2
O s
)
µ
H
16
2
O s
H
16
2
O m
H
3/2
18
2
O
H
18
2
O m
H
3/2
16
2
O
• We see the partition function ratio is temperature dependent
(which arises only from the vibrational contribution: temperature canceled in other modes).
• We also see that we can predict fractionations from measured vibrational frequencies and atomic and molecular masses.
Temperature Dependence
• The temperature dependence is:
•
Q vib
= e
h n
/2 k T
1
e
h n
/k T
• At low-T (~surface T and below), the exponential term is small and the denominator approximates to 1.
• Hence
Q
» e
h n
/2k T h n and the fractionation factor can be expressed as vib
=
1
-
2k T a =
A
+
B
T
• At higher temperature, however, this approximation no longer holds and α varies with the inverse square of T: a =
A
+
B
T
2
• The temperature dependence leads to important applications in geothermometry & paleoclimatology
O isotope fractionation between CO
2 and H
2
O
Kinetic Fractionations:
Reaction Rates
• Looking again at the hydrogen molecular bond, we see it takes less energy to break if it is H-H rather than D-H.
• This effectively means the activation energy is lower and the rate constant, k, will be higher: DH will react faster than H
• We can calculate a kinetic equilibrium constant from the ratio of rate constants:
2
.
k
A
= a k
B
• This will make no difference is the reaction goes to completion, but will make a difference for incomplete reactions. (good example is photosynthesis, which does not convert all CO organic carbon).
2 to
Kinetic Fractionation:
Diffusion
• Lighter isotopic species will diffuse more rapidly.
o Energy is equally partitioned in a gas (or liquid). The translational kinetic energy is simply o E = ½mv 2 .
o Consider two molecules of carbon dioxide, 12 C 16 O 2 and 13 C 16 O
2
, in a gas.
If their energies are equal, the ratio of their velocities is (45/44) 1/2 , or 1.011.
Thus 12 C 16 O
2 can diffuse 1.1% further in a given amount of time at a given temperature than 13 C 16 O
2
. But this applies to ideal gases (i.e., low pressures where collisions between molecules are infrequent. o For the case of air, where molecular collisions are important, the ratio of o the diffusion coefficients of the two CO roots of the reduced masses of CO
2
28.8):
D
12
CO
2
D
13
CO
2
=
µ
12
CO
2
µ
13
CO
2
air
air
=
2 species is the ratio of the square and air (mean molecular weight
4.1906
4.1721
=
1.0044
o leading to a 4.4‰ fractionation (actually observed).