What is a buffer
advertisement
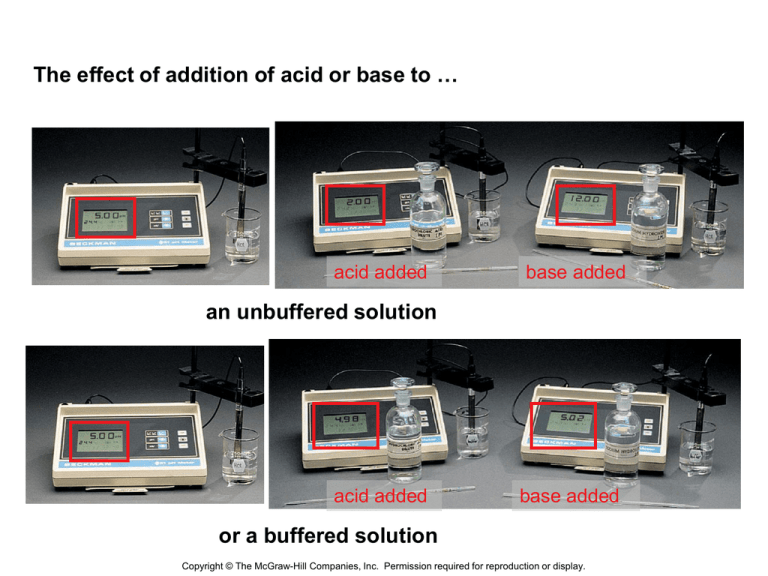
The effect of addition of acid or base to … acid added base added an unbuffered solution acid added base added or a buffered solution Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Characteristics of Buffers • 1. Contains a weak acid HA and its CB A• 2. Resists changes in pH by reacting with any added H+ or OH-, so these ions do not accumulate. • 3. Any added H+ reacts, • H+ + A- → HA • 4. Any added OH- reacts, • OH- + HA → H2O + A- What is a buffer? • Chemical system that can withstand pH changes when (limited) amounts of acid or base are added. • A buffer system consists of two components – acid component to react with OH1- ions – base component to react with H1+ ions • The components should not consume each other • A solution containing both: a weak acid + its salt OR a weak base + its salt. • Works on Le Châtelier’s principle and Common Ion Effect Common Ion Effect • A solution of two dissolved solutes that contain the same ion - common ion e.g. In solution of CH3COOH + CH3COONa CH3COOH(aq) <-----> CH3COO1- (aq) + H1+ (aq) CH3COONa (s) + H2 O(l) ---> CH3COO1- ( aq) + Na1+(aq) Both solutes produce CH3COO1- ( aq) ions. • According to Le – Chatelier’s principle, if add acid, then reaction HA <---> H+ + A– goes to left to absorb change; vice-versa if add base Figure 19.2 How a buffer works Buffer after addition of H3O+ Buffer with equal concentrations of conjugate base and acid H3O+ H2O + CH3COOH H3O+ + CH3COO- Buffer after addition of OH- OH- CH3COOH + OH- H2O + CH3COO- Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Buffering capacity: amount of H+ or OH- a buffered solution can absorb without significantly changing the pH. Capacity determined by magnitude of [HA] and [A-]. Concentrations of the components should be high enough to neutralize the added H1+ or OH1- ions Buffers in Natural Systems •Biological fluids, eg blood, contain buffers: pH control essential because biochemical reactions are very sensitive to pH •Human blood is slightly basic, pH 7.39 – 7.45 •In a healthy person, blood pH is never more than 0.2 pH units from its average value •pH < 7.2, “acidosis”; pH > 7.6, “alkalosis” •Death if pH < 6.8 or > 7.8 Buffer System in Blood “extracellular” buffer (outside cell) H+ + HCO3– < --> H2CO3 < -- > H2CO3 H2O + CO2(g) –Removal of CO2 shifts equilibria to right, reducing [H+ ], ie, raising the pH –The pH can be reduced by: H2CO3 + OH– <--- > HCO3– + H2O Another Blood Buffer •phosphate buffer, present inside cells (“intracellular” buffer) •H2PO4– and HPO42– : H2PO4– < -- > H+ + HPO42– from H3PO4 , a tribasic (triprotic) acid Figure 19.3 The relation between buffer capacity and pH change Problem: Which of the following solutions are buffers? a) KH2PO4/H3PO4 b) b) 0.30 mol CH3COOH/ 0.30 mol NaOH • Solution: • a) H3PO4 is a weak acid and KH2PO4 , a salt of its conjugate base H2PO4 1-, is a weak base. The system contains a conjugate acidbase pair and therefore is a buffer system b) 0.30 mol CH3COOH/ 0.30 mol NaOH Stoichiometric quantities of a weak monoprotic acid and strong monohydroxy base will produce a salt containing the conjugate base of the weak acid, CH3COONa. This weak base alone without its conjugate acid will not be a buffer. Auto-ionization of water • 2 H2O ↔ H3O+ + OH• This solution is still neutral b/c there are equal amounts of [H+] and [OH-], which is 1.0 x 10-7 M Indicators and Titration Curves Acid – Base Titrations • is a carefully controlled neutralization reaction • acid + base salt + water • involves the progressive addition of one reactant from a burette, to a known volume of the other reactant in a flask. • Standard – solution in which you know the concentration Acid-Base Indicators • Indicators - Weak organic acids or bases – Distinctly different colors in two forms – Two forms related to pH of solution – Phenolphthalein – most often used indicator for titrations • Clear – acidic • Pink – basic Acid – Base Indicators • HIn (aq) H+(aq) + In-(aq) • In acidic medium, equlibrium shifts to the left and the predominant color is HIn color In basic medium, equilibrium shifts to the right and the predominant color is In1- color • Colors and approximate pH range of some common acid-base indicators The color change of the indicator bromthymol blue basic acidic change occurs over ~2pH units Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Titration • Animation Equivalence / End Pts • Equivalence point – when neutralization occurs # H+ ions donated = # H+ ions accepted or total moles H+ ions = total moles of OH- ions • End Point – when the indicator changes color Strong Acid-Strong base Titration •pH starts low and increases gradually as acid is neutralized by the added base •Close to equivalence point pH rises steeply •Beyond this, pH increases slowly with addition of more base •Equivalence point • the mol OH1- = mol H1+ •The pH = 7 Strong base titrated with a strong acid Equivalence pt is 7 Strong acid titrated with a strong base Weak Acid-Strong Base Titration •Initial pH of weak acid •starts higher than pH of a strong acid of the same molarity •increases as the acid is neutralized by added base •Gradually rising curve is called buffer region as the weak acid reacts with the strong base to produce its conjugate base •At the mid-point of buffer region half of original acid has reacted with the base to produce conj. base Strong base titrated with a weak acid Equivalence pt above 7 Weak acid titrated with a strong base Weak Base-Strong Acid Titration •Initial pH of weak base •starts higher than 7 but lower than pH of a strong base of the same molarity •Decreases as the weak base is neutralized by added acid •Gradually dropping curve is called buffer region as the weak base reacts with the strong acid to produce its conjugate acid •At the mid-point of buffer region half of original base has reacted with strong acid to produce conj.acid Weak base titrated with a strong acid Equivalence pt below 7 Strong acid titrated with a weak base Steps to a Titration • Determine what indicator to use based upon strengths of acid/base & known end points for indicators • Fill a buret with standard solution & record initial volume • Start adding std solution slowly, with mixing, to the solution of unknown concentration • Continue adding std solution until equivalence point in reached (equivalence & end point should be relatively close) • Utilize given and obtained data to determine concentration of unknown. Calculations • Write the balanced equation for the neutralization • Extract all the relevant information from the question . • MAVA = MBVB – This can be used because your moles of hydronium and hydroxide are equal at the equivalence point. – CAUTION: If the acid is polyprotic or the base is polyhydroxic, you must use the normality instead of molarity in this formula. • Normality is found by multiplying the number of hydronium or hydroxide ions in the formula unit by the molarity. Example A 43.0mL volume of NaOH was titrated with 32.0 mL of 0.100M HCl. What is the molarity of the sodium hydroxide solution? What key information can be obtained from problem? NaOH + HCl NaCl + H2O 43.0mL NaOH 32.0mL 0.100M HCl Use the formula and plug in the values. MAVA = MBVB (x)(43.0ml) = (0.10 M)(32.0 ml) M(HCl) = 0.075 M Example #2 A volume of 25 mL of 0.120 M H2SO4 neutralizes 41 mL of a NaOH solution. What is the concentration of the NaOH? What key information can be obtained from problem? 2 NaOH + H2SO4 Na2SO4 + 2 H20 25 ml, 0.120M H2SO4 41ml, NaOH Use the formula and plug in the values. MAVA = MBVB (0.120M x 2)(25 ml) = (x)(41 ml) M(NaOH) = 0.14 M Your turn…. 1. If it takes 54 mL of 0.1M NaOH to neutralize 125 mL of an HCl solution, what is the concentration of HCl? 2. It takes 25 ml of a 0.05M HCl to neutralize 345mL of NaOH solution, what is the concentration of the NaOH?






