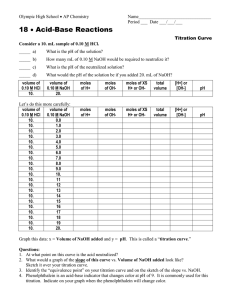
Titration Curve Mathematics: Strong Acid/Base Titration
advertisement

THE MATHEMATICS IN A TITRATION CURVE (WITH A LITTLE BASE 10 AND LOGARITHM ARITHMATIC ADDED) INTRODUCTION OF BASIC CONCEPT OF TITRATION CURVES USING TITRATION OF STRONG ACID WITH STRONG BASE How do calculations of pH where the solution changes from acidic to basic compare to the curve of the titration graph? Recall: What is a mole? 6.022 x 10 23 molecules, atoms, or ions # M represents the fraction ( # moles)/(1 liter of solution) and this value tells us the concentration of the solution pH = -- log [H+] An example of a strong acid base reaction is: Add drops of NaOH To a solution of HCL --Add an indicator which changes color with pH (phenalthalien turns from clear to pink) Background Concepts: Strong Acid titrated with a Strong Base Consider the titration of a 50ml sample of 1M HCl titrated with 1M NaOH Add drops of NaOH To 50 ml of 1M solution of HCL 3 important Calculations of pH when the volume of NaOH added is close to Equivalency Point: ml of NaOH = 49.99 ml or 0.04999 liters total volume in beaker is approximately 100 ml There is 0.01 ml of H+ ions which have not paired with OH- to form water. [H+] = 10 ^ -4 pH = 4 When the ml of NaOH = 50 ml then total volume of added NaOH equals volume of HCl all H+ ions have combined with OH – ions to form water neutral pH = 7 note: K w = = [H+] [OH-] for this + volume of NaOH ---- [H ] =[OH ] giving the equation K w =[H+] 2 and [H+] = 10^-7. This reviews laws of exponents – taking square root means dividing exponent by 2. When ml of NaOH added = 50.01 ml There is 0.01 ml of excess OH— ions in solution. [OH-]= 10^-4 Since [H+] [OH-] = 10^-14 then [H+] = 10^-14 / [OH-] [H+] = 10^-14/10^-4 or [H+]= 10^-10 and pH = 10 CONCLUSION: The pH changes 6 points when the volume of NaOH changes 2 ml close to the equivalency point for this mixture of strong acid and strong base. Relate this to the titration curve for strong acids and strong bases.